Foreword
Electrocardiogram (ECG) is the easiest way to examine cardiac problems. There are many ECG device developer devoted to offer sophisticated and accurate ECG devices for medical institutions and public. Before the government permits to release those ECG devices to the market, every country does have an official institute to assure the quality and performance of those ECG devices.
However, ECG device developers are often unsure about when to conduct the validation test. This article provides a test method for the timing and strategy of conducting the effective validation test by using proper test equipment.
1. Under What Circumstances You Shall Conduct Validation Test for ECG Device
The life cycle of ECG devices shall consist of ‘‘launched’’ and ‘‘in development’’.
Let’s start from when the launched ECG device should again undergo validation test. Such products are already consistently in either performance or functionalities followed the guideline of ISO13485 (Medical Device Quality Management System). Then, when would we need to conduct validation test? It shall be at the time when the design is changed to different function blocks as listed below. The validation is to make sure the changes are effective and not degrade the overall performance.
- Hardware component replacement
- Firmware version update
- Algorithm version update
For ECG devices in development, A completed system validation test should be conducted whenever a sample is produced, or a version is released to ensure all the modifications are complied with expected specification.
2. How to Conduct the Validation Test
Realistic ECG signals are strongly recommended along with the required tests defined in IEC international standards. Realistic ECG signals can be obtained in public databases. On top of that, distinguished ECG signals recorded by previous versions are even more important. With those public or recorded waveforms, we would be able to compare the subtle differences between the revisions.
As for the test equipment, WhaleTeq MECG 2.0 test system can convert digitally-recorded ECG waveforms for up to 8 channels output (equal to 12 leads) in nearly loss-free analog signals (click link for details). The analog ECG signals will be repeatedly output to current and new versions of the ECG device (see Figure 1) and the ECG device shall save the test waveforms in digital files for further analysis.
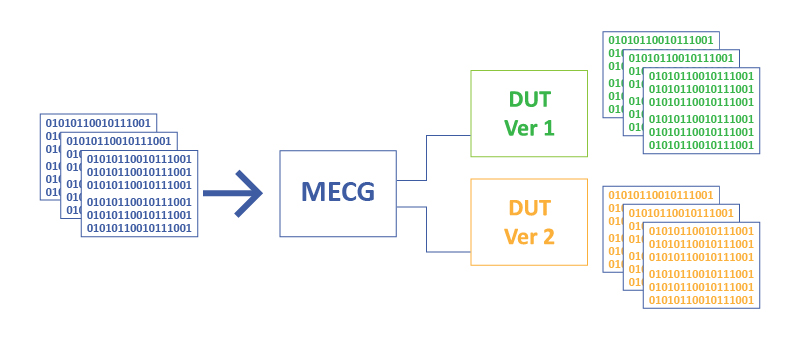
Figure 1. Use MECG 2.0 to repeatedly output the waveforms in analog signal
Correlation analysis then can be proceeded based on the collected signals from the current and new version PCBA. The result of the PQRST waveform measurements can also be used to analyze the variation of each parameter.
A. Correlation Analysis Example
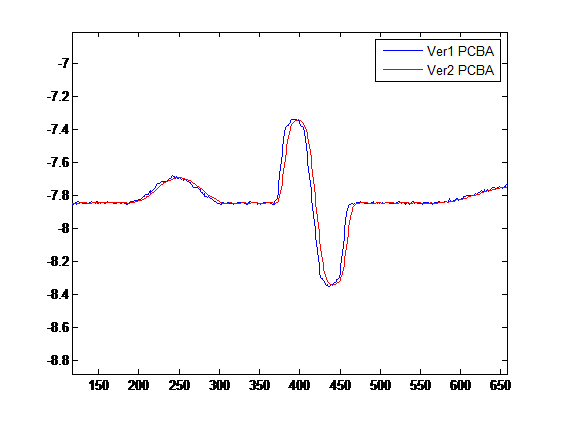
R= 0.953
B. ECG Waveform Measurement Example
| Current Version | New Version | Difference | |
| P duration | 116ms | 117ms | 1ms |
| P amplitude | 0.15mV | 0.148mV | -0.002mV |
| QRS duration | 112ms | 112ms | 0ms |
| Q amplitude | -0.1mV | -0.08mV | 0.02mV |
| R amplitude | 1.03mV | 1.04mV | 0.01mV |
| S amplitude | -0.15mV | -0.14mV | 0.01mV |
| ST80 amplitude | 0.05mV | 0.04mV | -0.01mV |
| QT interval | 350ms | 352ms | 2ms |
| T amplitude | 0.5mV | 0.51mV | 0.01mV |
According to the correlation analysis and ECG waveform measurements, we can determine the pass criteria of the validation test and confirm whether the performance of the new version PCBA can meet the design expectation.
Conclusion
Conducting repetitive validation tests via WhaleTeq MECG 2.0 test system can easily assure the validation process for each revision are completed. In this way, we would be able to improve the functionality while make sure system performance is not downgraded.
