To comply with international medical regulations, the introduction of Unique Device Identification (UDI) system is currently the most important and urgent task for medical device manufacturers. From the generation of UDI barcodes, label printing and packaging to data management, all items are interconnected and indispensable. It is a huge project that spans various departments and professions.
This article will provide a detailed explanation on the UDI importing process and provide solutions to help optimize this process.
- Common processes of importing UDI
Building and importing UDI is a lengthy process. First of all, the process must comply with the quality management system/ISO13485 standard and write relevant second-, third- and fourth-level documents.
After confirming the plan, the company usually needs to complete the following steps in order:
- Select number issuing authorized organization: Choose according to the actual situation of the enterprise. Currently, the three major organizations are GS1, HIBCC and ICCBBA.
- Create Device Identifier (DI): Create according to the standards of the above and determine the composition of the production identifier (PI).
- Medical device registration: registration, registration change or filing, the registrant/filer should submit Device Identifier (DI) in the registration/filing management system.
- Select data carrier*: Give the medical device a single identification data carrier on the smallest sales unit of the medical device and higher-level packaging or medical device products.
- Upload UDI information to the national database: Before the product is put on the market, product identification and related information should be uploaded to the UDI national database.
- Regularly update the database: When product identification and related information change, update it to the national database in a timely manner.
* Note 1: Data carrier refers to the medium or platform that stores and transmits digital information.
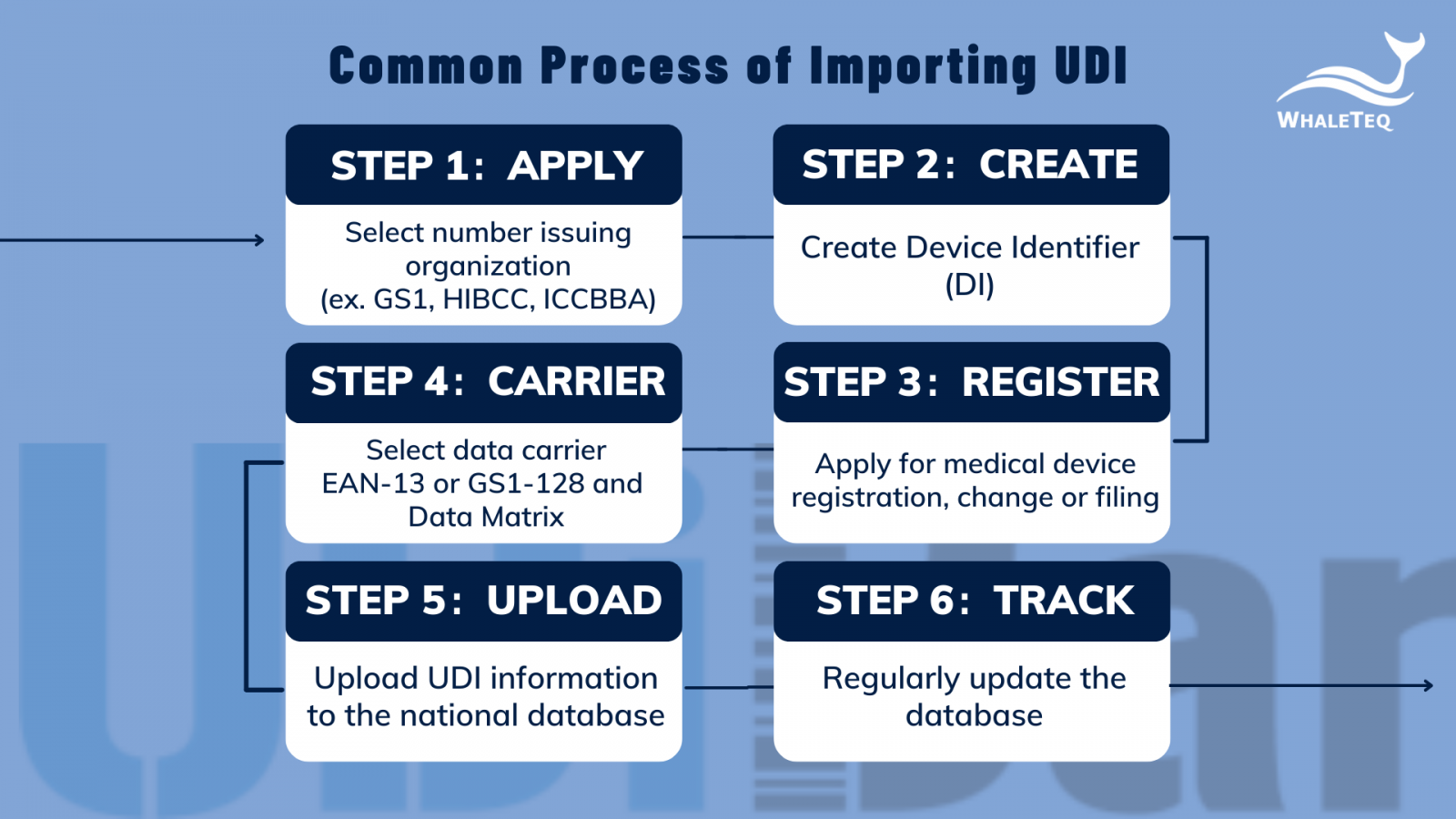
Figure 1: Common Process of Importing UDI
- Challenges and required resources for importing UDI
In addition to the fixed and mandatory barcodes for UDI system, enterprises should establish their own set of barcode standards and database system.
On the contrary, if the standards are not unified and multiple data management methods are dispersed, it will not be conducive to the exchange and sharing of information in subsequent links. It will also be difficult to find the person responsible during the traceability process.
- Corporate compliance
Generally speaking, large companies usually allocate professional teams to be responsible for the introduction of UDI system. These companies have complete teams and experience, human and financial resources, etc. They can independently register and review with the competent authorities of various countries and submit the DI information. The PI can be coded and managed by the enterprise itself. Compared with large enterprises, small and medium-sized manufacturers have more limited resources, experience and funds, and will face huge challenges when introducing UDI.
- Labor costs
Medical device manufacturers are the leaders in implementing UDI. Since it costs a certain amount of money to establish, companies need to edit their own PI codes. As the amount increases, there are also problems with data storage, query, circulation, application and other issues, which require a large amount of manpower and resources to complete. Although you can use Excel tables to store coded data by yourself, it is easy to make mistakes in writing records through Excel. For example, multiple departments handle the file during the file transfer process, resulting in data errors, omissions, inconsistent versions, etc., which will bring problems to subsequent inspections and auditing.
- Production process and equipment
In order to comply with the principles of barcode durability and label readability, medical device manufacturers should have the ability to produce production equipment and IT systems that meet standards, or to upgrade existing equipment and systems. For example: the production line should be able to print UDI label, and also need systems to check readability, such as vision systems, scanning systems, and verification systems.
In terms of production process, manufacturers must consider multi-department collaboration, because the implementation scope of UDI covers the entire process of raw material entry and inspection, product production, intermediate product control, finished product packaging, storage, transportation, production and quality management. The production and QA department should cooperate in establishing SOPs for operation and inspection, product storage and transportation processes, and even the update of product registration information.
- Strategies
Before the actual introduction, you can follow the following steps to plan the introduction process step by step to help the company achieve its goals more efficiently.
- Determine the goals: Before importing UDI, the import goals should be clearly defined, such as: improving user safety, improving production efficiency, complying with regulations, etc. Clear goals help improve the entire import process.
- Seek professional support: Importing UDI requires specific technical knowledge and professional support. Consider working with a UDI expert or software provider to ensure the introduction goes smoothly and complies with regulatory requirements.
- Review existing process: Review the existing production process to determine which steps need to be modified to comply with UDI requirements. This may involve processes such as changing product labeling, tracking inventory, or modifying data management.
- Choose the technology consistent with production process: Like barcode scanning, RFID technology or other automatic identification technologies, to ensure that the selected technology can meet the needs and is scalable.
- Data management: Consider how UDI data is managed and stored. A reliable data management system should be in place to query and track data when needed.
- Monitoring and improvement: After UDI is introduced, the process should be continuously monitored to check for problems or opportunities for improvement. This helps ensure UDI validity and compliance.
- Solution
WhaleTeq develops a dedicated UDI Generator and Label Management System-UDiBar. Its design follows regulations and standards , from front-end barcode editing, national database upload, automatic introduction of production information, label design and printing, to product post-market tracking management, only requires a single software platform to complete all workflows.
In addition, UDiBar has an authority review and control mechanism and provides a software validation report that complies with ISO13485 quality management requirements, which can effectively shorten the introduction time and reduce personnel training troubles.

Figure 2: UDiBar Product Structure
The following explains how UDiBar actually solves UDI importing problems in accordance with the international standard GS1:
1. Adopt GS1 coding principles
GS1 is a global standards organization that provides a widely used coding system for identifying products, locations, assets, etc. Manufacturers need to choose a coding method that complies with GS1 standards to ensure the unique identification of their products.
UDiBar has built-in Device Identifier (DI) verification code calculation and checking functions, and can verify whether product codes are reused to ensure the correctness and uniqueness of each DI.
Besides, Production Identifier (PI) can also be defined, automatically sorted according to rules such as fixed information first and changing information last, and the changing information is automatically brought into label printing, reducing human errors and realizing automated production needs. The output UDI code is completely recorded in the database to ensure that the PI is accurate and can be traced back to the specific production process.
2. Adopt GS1 barcode standards
GS1 provides a set of barcode standards to convert UDI codes into scannable barcodes. Manufacturers should follow GS1 barcode standards to generate UDI barcodes and ensure readability and global versatility.
UDiBar converts UDI encodings into machine-readable barcode types. Comply with the GS1 application identification code standard barcode GS1-128 (ISO/IEC 15417) and Data Matrix (ISO/IEC 1 6 022) to ensure that the barcode can be correctly decoded when scanned and can be used globally.
Users can edit labels in UDiBar, including UDI barcodes, text messages and other necessary identification elements. Label design should comply with GS1 standards to ensure consistency. All produced labels have complete records, and the printing records and corresponding base images can be traced back at any time.
3. Barcode quality control
Manufacturers should implement quality control procedures to ensure that the barcodes generated are of good quality and do not suffer from scanning issues. This includes the clarity, contrast, size and position of the barcode. UDiBar is designed with a code scanning detection function. Connect a code scanner to perform a code scan to confirm whether the barcode printing quality can be read. It also checks whether the information carried in the barcode is the UDI code produced by UDiBar, to achieve quality control and data inspection.
4. Data integration and hierarchical management
Set admin and user access limitation according to the responsibilities of the legal, quality control, and manufacturing departments. Information must be reviewed and approved by admin before it can be used, effectively ensuring accuracy.
UDiBar can integrate all UDI data into the same database to avoid errors when converting software and copying information across files. At the same time, the reviewed UDI data can be directly imported into UDiBar for label editing, eliminating the time of copying and pasting.
Optimizing the UDI importing process requires planning, execution, and continuous improvement. Through clear goals, professional support and training, small and medium-sized enterprises can achieve effective introduction of UDI, thereby improving product quality, complying with regulatory requirements and improving competitiveness.