For ECG device manufacturers, the most critical phase before product launch—following design, development, and verification—is clinical trials.
The clinical trial process generally consists of four steps: preparation before the trial, setting clinical trial objectives and protocols, executing the trial, and completing data analysis before submitting reports to regulatory bodies.
Since clinical trials involve a considerable number of subjects and must be conducted in various manufacturer-defined environments, they demand significant investment in time and resources. Therefore, adequate preparation can help minimize repeated trials, ultimately reducing costs and expediting production and time-to-market.
Challenges in Preparing for Clinical Trials
1. Meeting Medical Electrical Equipment Standards
Before ECG devices can detect ECG signals, they must first meet fundamental electrical specifications.
These specifications define amplitude accuracy, dynamic range, frequency response, and noise processing capabilities, with reference values and allowable tolerance outlined in international standards such as IEC 60601-2-25 and IEC 60601-2-47.
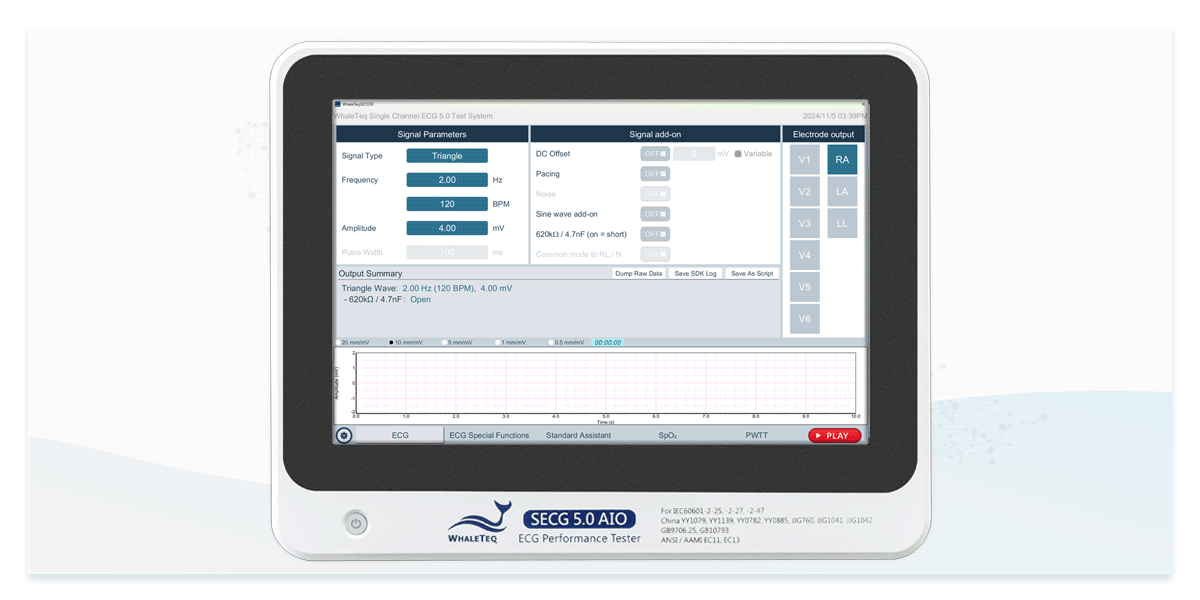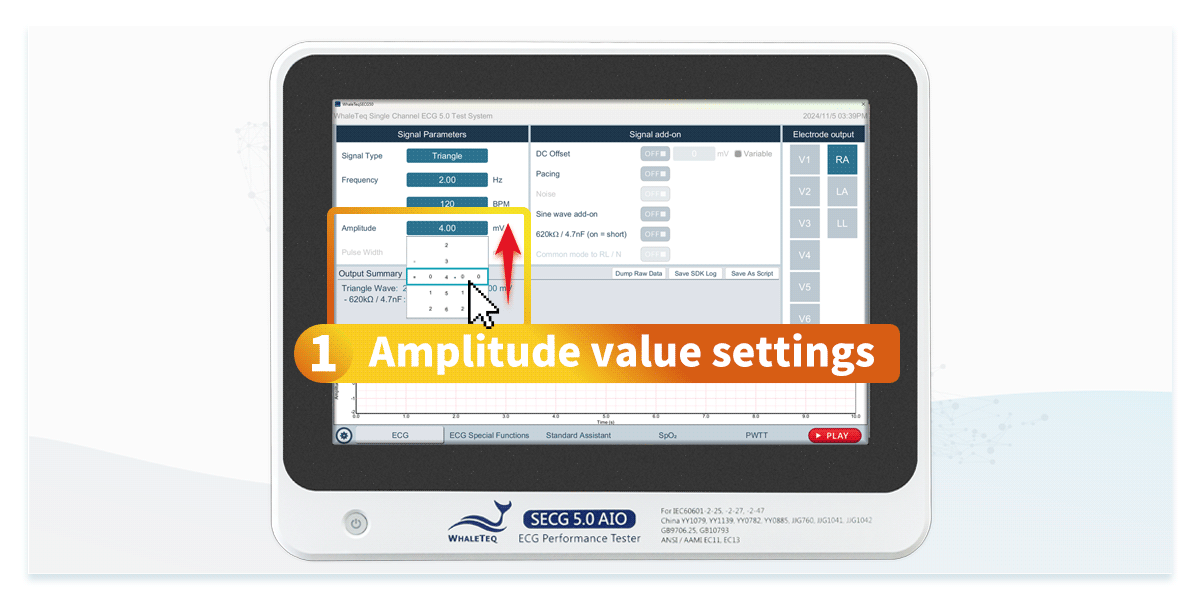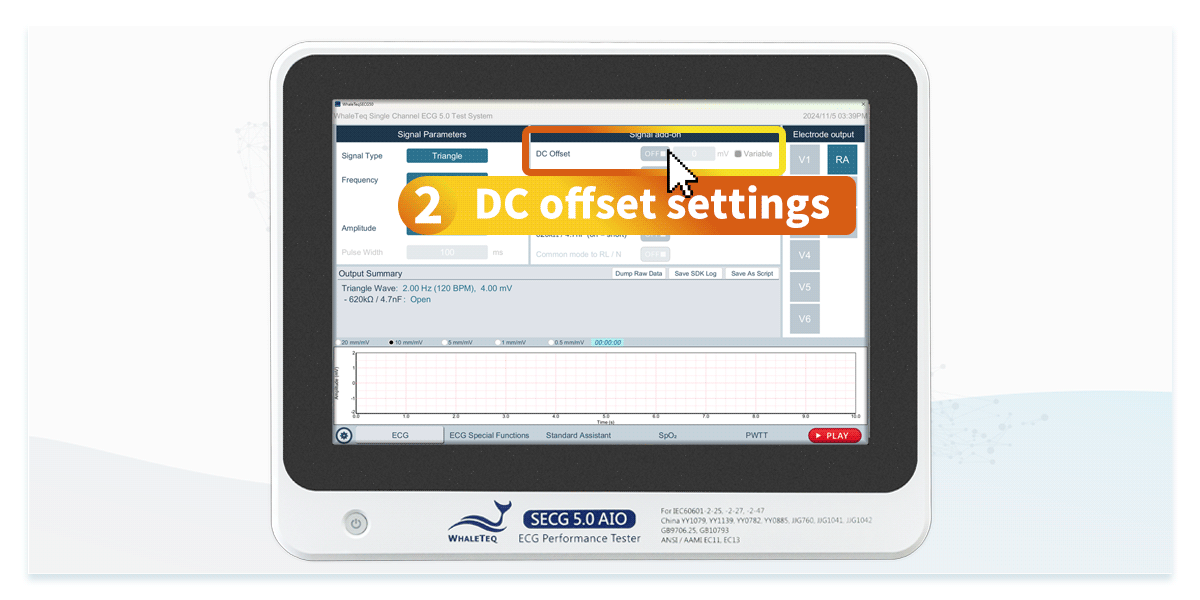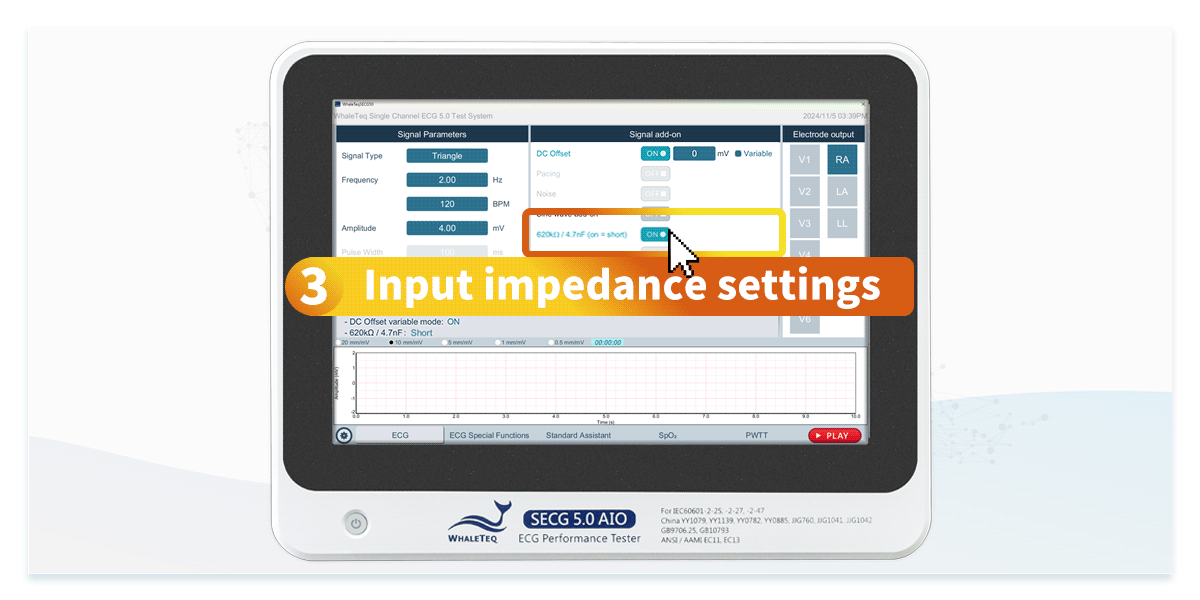
Compliance tests primarily use standard signals, such as fixed-frequency sine and triangle waves, to evaluate an ECG device's fundamental performance.
Only when an ECG device meets the required amplitude and time accuracy can it advance to the next stage: measuring real ECG signals.

2. Performance in Real ECG Signal Measurement
Once the ECG device passes fundamental compliance tests, the next challenge is its ability to accurately interpret real ECG signals.
Unlike standard signals used in compliance testing, real physiological signals are inherently unstable, posing a challenge for ECG devices. If an ECG device misinterprets or fails to recognize possible symptoms represented by the ECG signals, health professionals may not receive accurate information for proper diagnosis and treatment.
Additionally, if a patient has a cardiac pacemaker, the ECG device must faithfully detect and display pacemaker pulses to ensure they are not confused with ECG signals.
How to Overcome These Challenges?
Similar to preparing for an exam, conducting mock tests allows engineers to identify and address weaknesses before the official clinical trial.
By testing ECG devices' performance and algorithms with standard signals and clinical data in advance, manufacturers can ensure that their devices are ready for clinical trials, reducing unnecessary retesting and associated costs.
1. How to Ensure Compliance with Particular Requirements for the Basic Safety and Essential Performance of Medical Electrical Equipment—Verifying Design with an ECG Simulator
ECG signals differ significantly from those generated by general-purpose signal generators. Using general-purpose signal generators for testing ECG devices may result in insufficient or imprecise signal output, and requiring engineers to develop additional test equipment to verify ECG devices would be a time-consuming process.
Using an ECG simulator specifically designed for ECG device testing can efficiently verify that specifications comply with standards. ECG simulators not only generate standard signals but also provide test signals required by IEC 60601-2-25 and IEC 60601-2-47, such as DC offset of ±300mV, input impedance of 620kΩ, and mains frequency noise at 50Hz and 60Hz. This allows engineers to accelerate compliance testing by configuring standardized test settings.
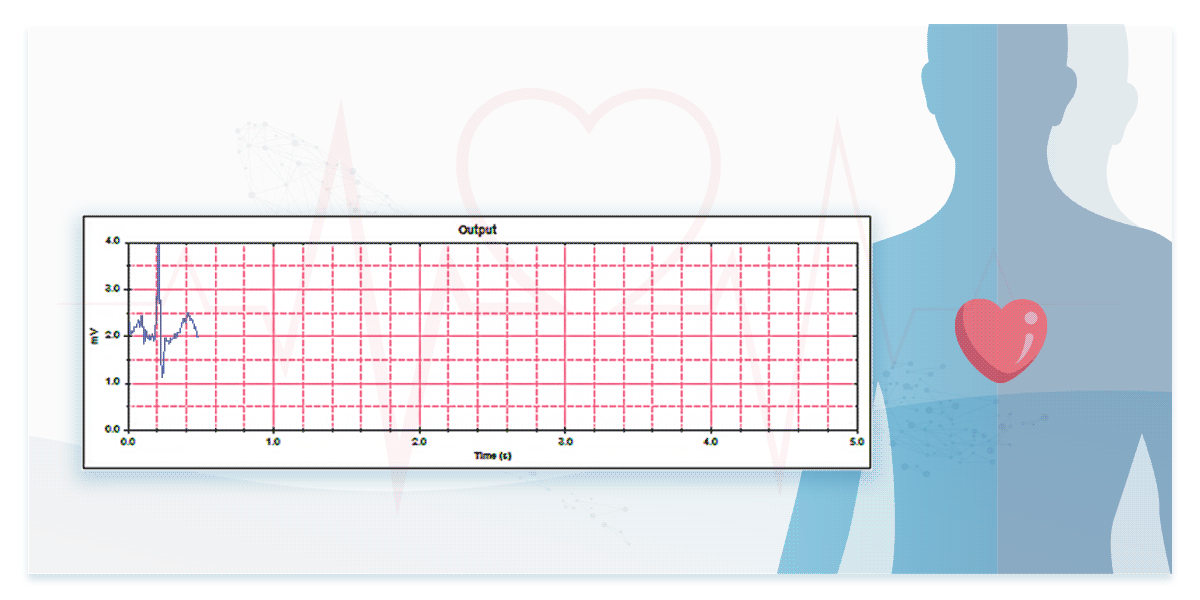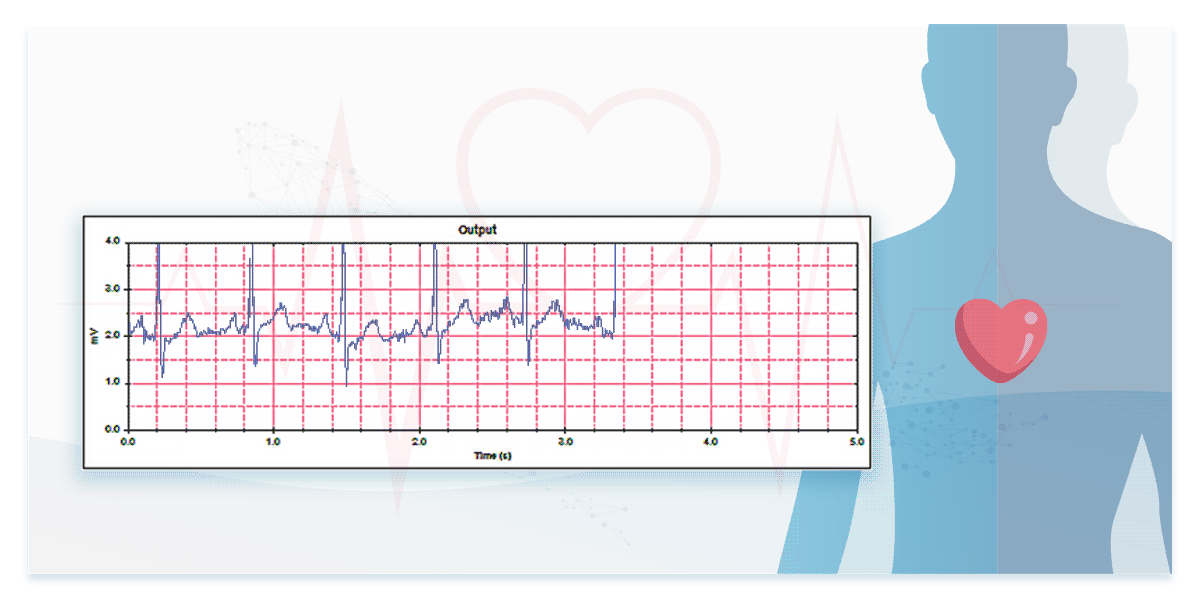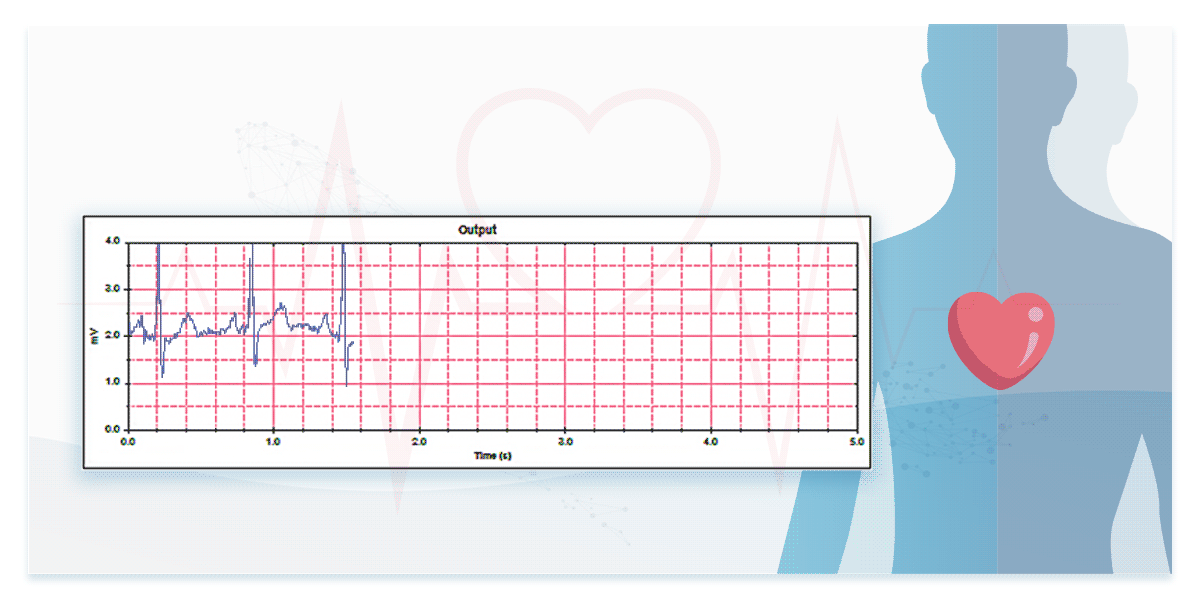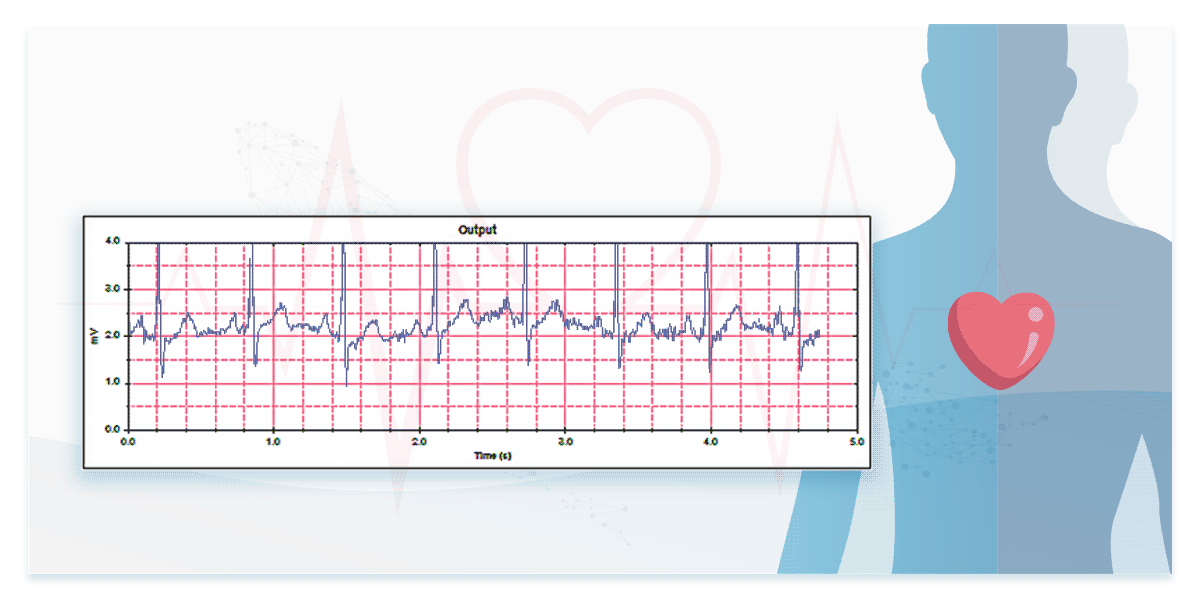
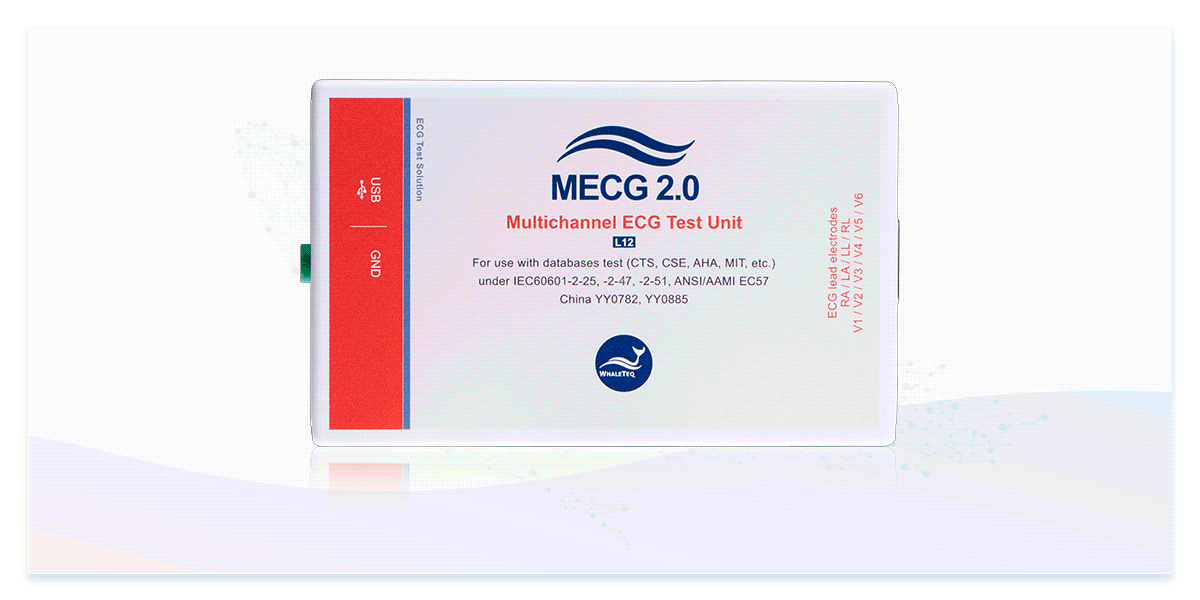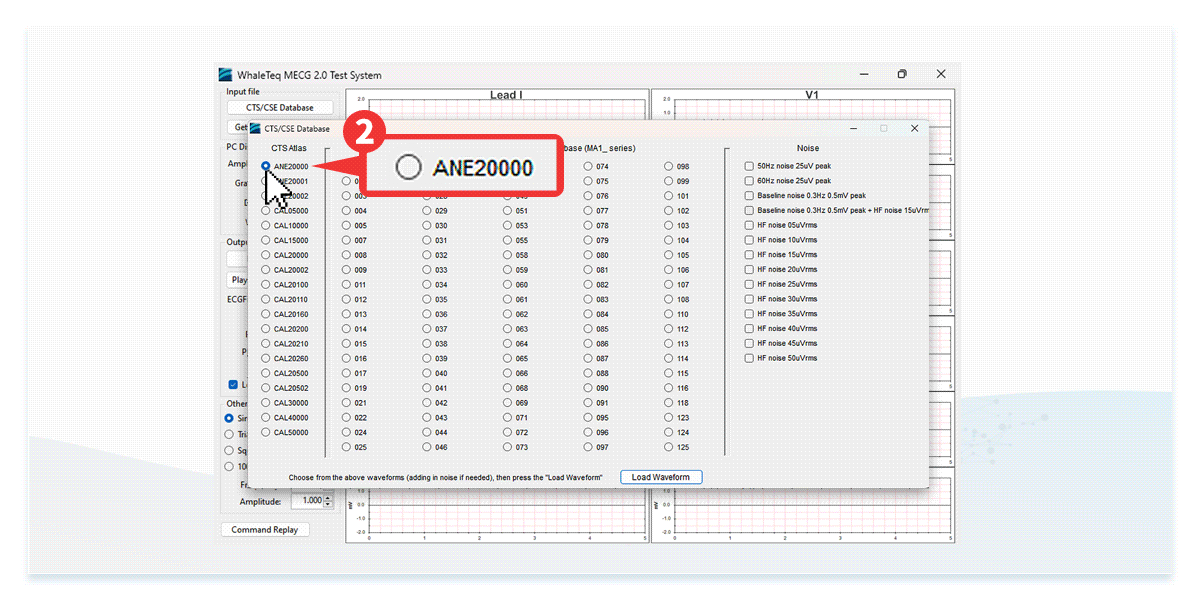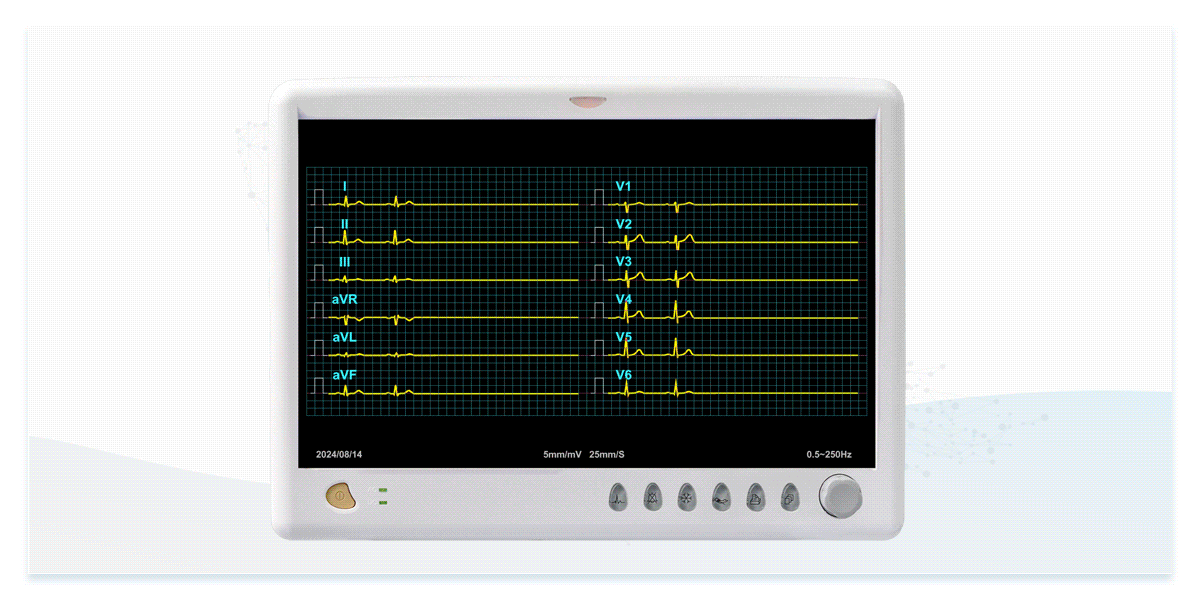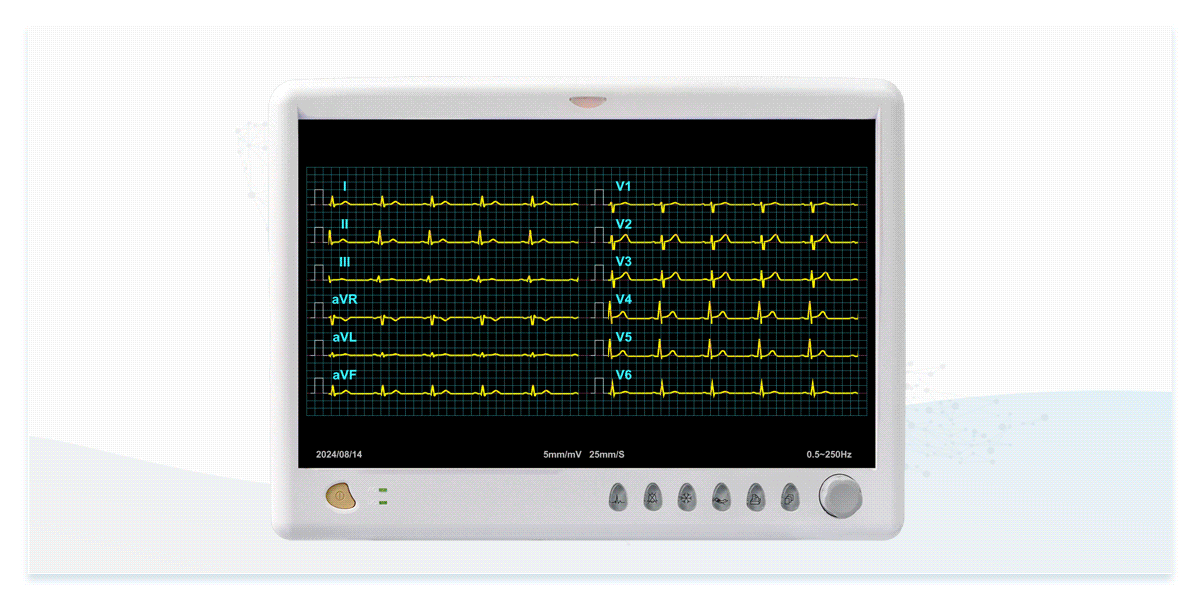
2. Performance in Real ECG Signal Measurement—Testing with ECG Databases
To assess performance in measuring real ECG signals, engineers must test devices using ECG databases.
Some international standards for ECG devices require database testing to evaluate ECG interpretation capabilities. For instance, IEC 60601-2-25 mandates the use of CTS and CSE databases, while IEC 60601-2-47 specifies AHA, MIT-NIH, and NST databases. Additionally, manufacturers can access and use publicly available resources such as the PhysioNet Non-Invasive Fetal ECG Database providing ECG data of both mothers and fetuses.
As with compliance testing, ECG database playback can be conducted using dedicated ECG simulators, eliminating the need for manufacturers to develop custom playback devices, along with the associated maintenance and calibration.
The dedicated ECG simulators are designed to prevent signal distortion and time difference, accurately reproducing real ECG waveforms to verify the interpretation capabilities of ECG devices for both single-lead and multi-lead configurations.
As ECG technology advances, computerized ECG analysis algorithms are becoming more prevalent, prompting the development of the IEC 80601-2-86 standard to include them.
Regardless of ECG technological advances, accuracy and consistency remain paramount in clinical applications. Beyond robust product design, clinical trials play a crucial role in ensuring ECG device readiness for real-world use.
In addition to compliance testing, manufacturers can optimize device performance ahead of clinical trials by utilizing ECG databases and clinical data for preliminary testing, ensuring devices are fine-tuned for optimal accuracy and reliability.
Suggested Products
A standalone simulator ideal for hardware and system engineers in the R&D department.
It generates single-channel ECG analog signals with options to superimpose respiratory, pacing, and noise simulated signals, closely simulating real-world conditions.
The simulator also supports playback of single-channel clinical data to assist in ECG device performance testing.
Designed specifically for ECG database and clinical data playback, the MECG 2.0 supports multichannel output for verifying the system and algorithm performance of 12-lead ECG devices.