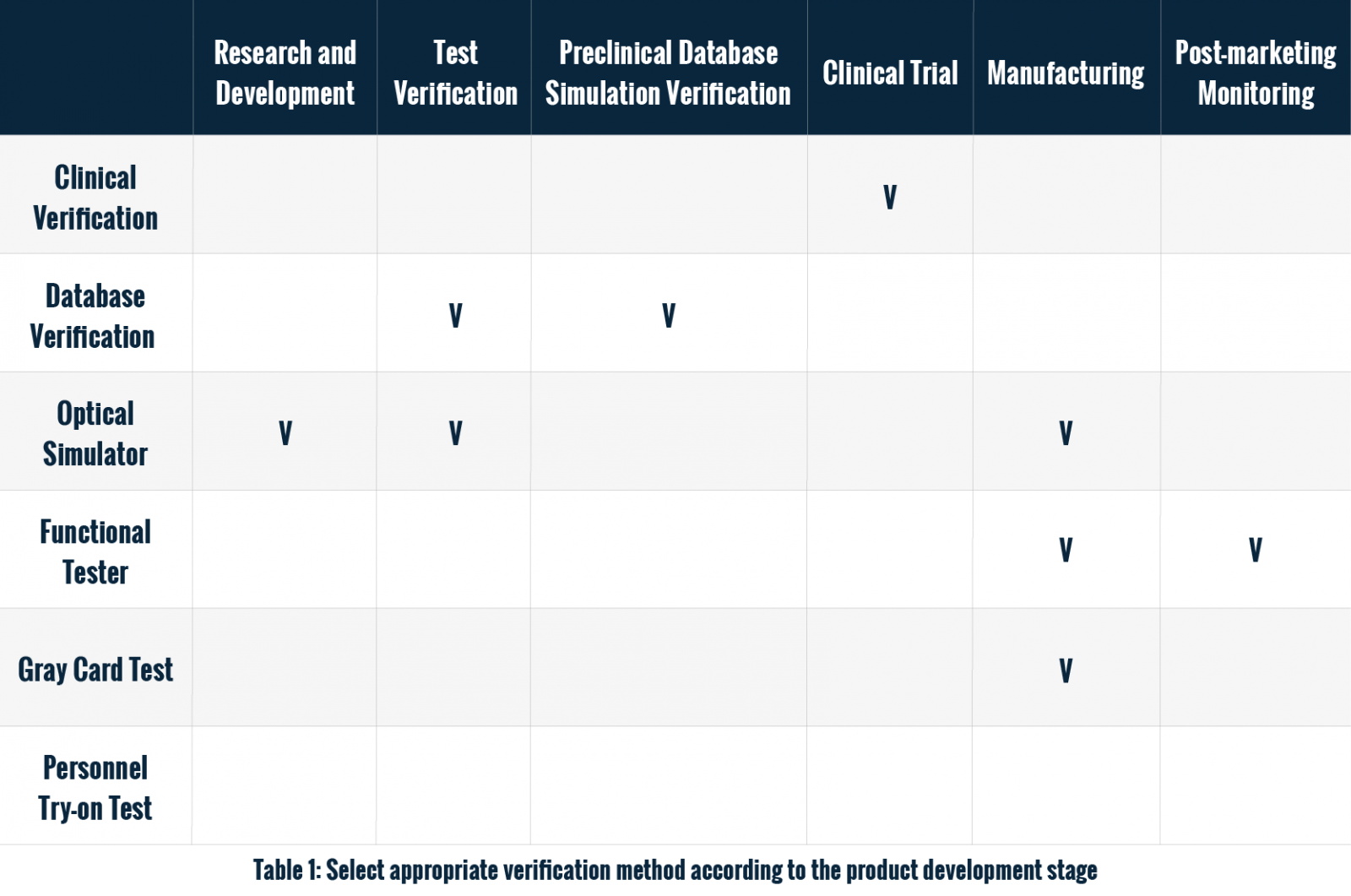
Introduction of PPG-Based Wearable Devices
With the popularity of wearable health monitoring devices, heart rate and blood oxygen monitoring functions based on photoplethysmography (PPG) technology have become the core of many products. PPG technology monitors blood flow changes through optical sensors to calculate heart rate and blood oxygen saturation (SpO2). However, to ensure that these devices remain reliable and effective in a variety of environments and conditions, verifying their performance is critical.
Choosing the appropriate verification method is not only related to the performance of the device in actual use, but also affects the cost and efficiency of the development process. This article will explore several common verification methods for PPG-based wearable monitoring function and provide an analysis of their advantages and disadvantages to help test engineers find the verification solution that best suits their needs.
The Theory and Techniques of PPG-Based Wearable Devices
The main theory of PPG technology calculates heart rate and SpO2 by emitting light waves to the skin and detecting the reflected light in blood vessels. When the heart beats, fluctuations in the blood flowing through the blood vessels will affect the intensity of reflected light. Based on these changes, the device can calculate the number of heartbeats and the concentration of oxygen in the blood.
In wearable devices, PPG technology is widely used, but its accuracy is affected by many factors, including skin color, motion interference, and user posture. These variables make how verification is performed critical.
Testing Methods for PPG-based Wearables
1. Clinical verification
Clinical verification is considered the most accurate method for verifying wearable devices since it directly compares results with medical equipment. This involves patients using the device, with heart rate data compared to an ECG machine and blood oxygen levels to a CO Oximeter.
The process also requires adherence to strict ethical and legal standards to protect patient safety and confidentiality, involving comprehensive research plans, sample size determination, and effective data collection methods.
Due to the high accuracy of clinical verification and its ability to cover a variety of medical conditions, it is trusted by medical professionals. However, the complexity and high cost of implementation, as well as the challenge of finding patients who meet various conditions in a short period of time for testing, make it difficult. As a result, many companies still seek supplementary methods for initial assessment during the product development phase.
Future advancements in technology and data analysis may enable more efficient, cost-effective verification methods to support medical device application in clinical settings.
2. Closed-loop testing - gray card
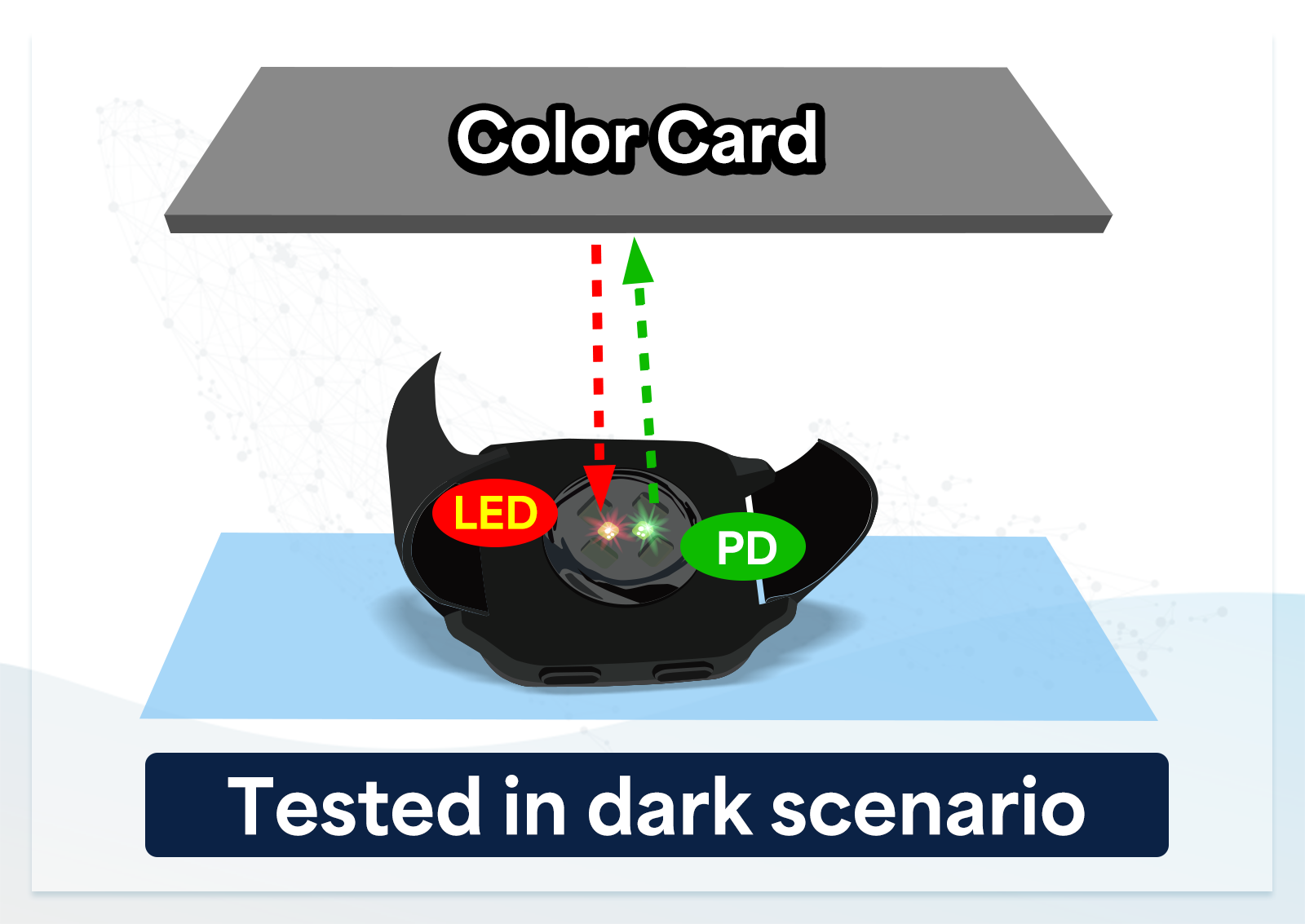
Gray card closed-loop testing is used to verify the overall performance of the LED and photodiode (PD) in wearable devices in terms of signal-to-noise ratio, ensuring the reproducibility of test results. This method reflects the LED signal from the gray card back to the PD in the closed-loop system to check if the device can correctly identify and process PPG signals.
However, it cannot pinpoint whether the LED, PD, or both are faulty, making it difficult to effectively filter out defective products or verify heart rate and SpO2 accuracy. Moreover, relying solely on the signal-to-noise ratio may overlook specific issues, such as the device's poor response under certain physiological conditions or data deviations.
3. Optical simulator - AECG100

The AECG100 is a multifunctional vital sign optical simulator, capable of simulating various scenarios such as different heart rates, blood oxygen levels, skin tones, and arterial elasticity. It aids developers in optimizing algorithms by replaying raw data, recreating clinical recordings, or custom-made waveforms to compensate for potential signal distortion due to analog-to-digital conversion, making it highly effective for testing in the research and development phase.
Additionally, AECG100’s rigorous validation process and comprehensive testing capabilities have made it popular with leading wearable device manufacturers. Although its optical interface may not meet the needs of all devices, customized fixtures can be designed to accommodate diverse testing requirements.
4. Database verification
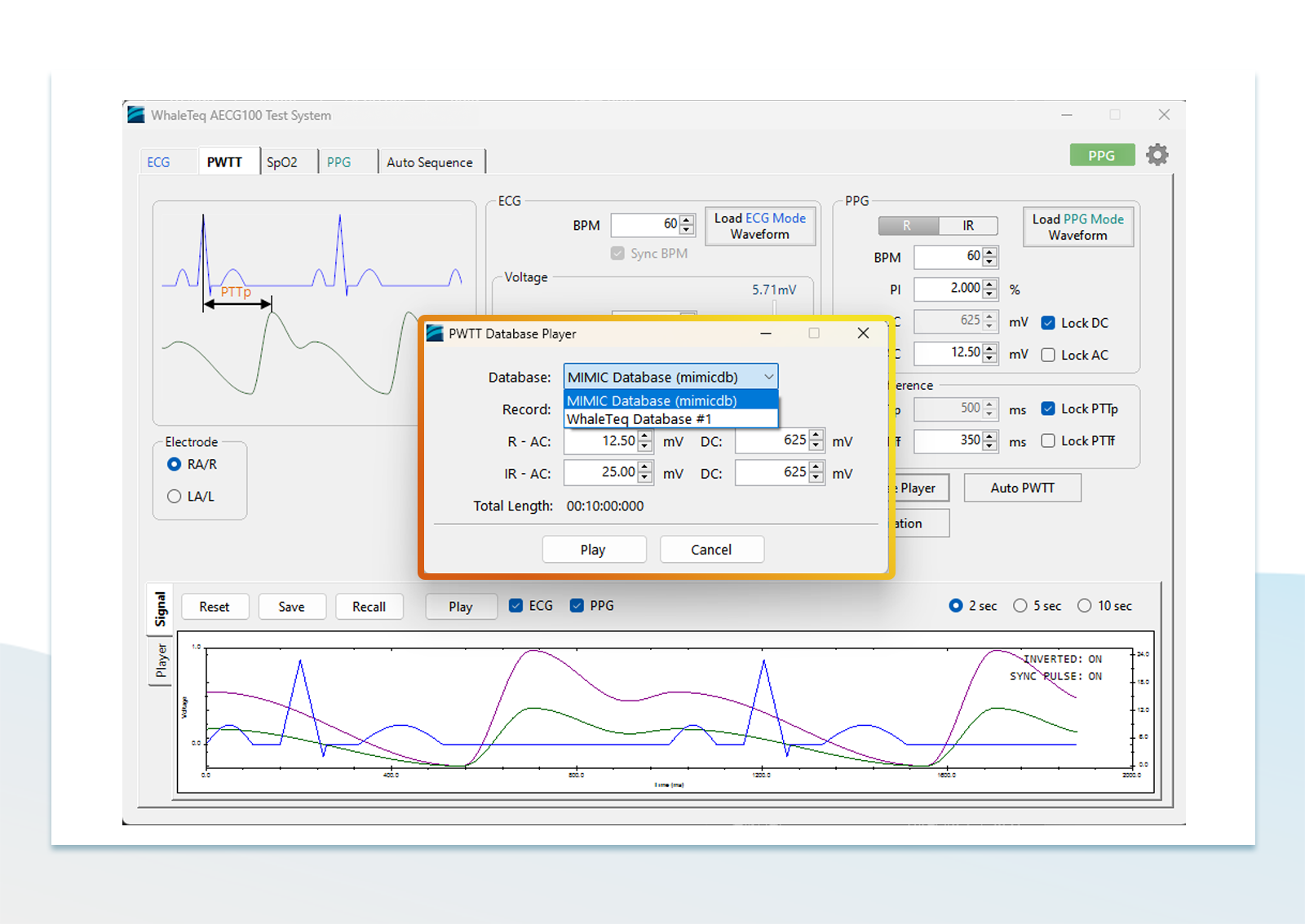
The database verification method mainly relies on a large amount of historical data for software testing, using digital signals to help verify the algorithm of the device, and this method has good scalability. However, in terms of data collection, in addition to taking a long time, how to collect a large amount and high diversity of data is also a big problem. In addition, through a signal simulator that can play back raw data, analog signals can be played back to confirm the device's performance in the real world.
5. Personnel try-on test

This method collects actual usage data by asking test subjects to wear wearable devices, and conduct tests according to different usage and activity scenarios, including: sleeping, exercising, working, etc. It should be noted that most testers are healthy individuals and difficult to test all patient conditions, which limits the breadth of testing.
Comparative Analysis
Each of the above methods is suitable for different stages of optical sensor development. In the early stage, optical simulators and database verification are effective options because they are less expensive and enable rapid repeat testing. In the later stage, clinical verification is required to ensure the accuracy and reliability of the wearables.
Challenges and Limitations
Although PPG technology is widely used in wearable devices, it still has some limitations.
For example:
- Technical limitations: Because the PPG signal is easily affected by factors such as ambient light, skin color, and blood vessel conditions, the accuracy of the data may vary under different environments.
- Optical compatibility: Compatibility between the wearable device under test and the optical simulator is critical to test accuracy. In order to improve accuracy, we need customized fixture designs for different types of wearable devices.
Future Trends
As artificial intelligence and machine learning technologies advance, these technologies are increasingly used in wearable device testing. The use of AI can improve the efficiency and accuracy of testing, and can better process complex data. In addition, future testing methods will be more inclined to combine multiple verification methods to fully cover different application scenarios.
Conclusion
Verifying PPG monitoring functionality in wearable devices is a complex task that requires the cooperation of multiple methods. From initial loop test to later clinical verification, each method has its scope of application, advantages and disadvantages. A verification strategy that combines multiple methods can help engineers balance accuracy, cost, and feasibility to develop more reliable wearable products.
Reference Products and Test Plans
AECG100 provides diverse data in different test environments and simulate various physiological and environmental conditions. Its advantages include:
- Broad simulation range: AECG100 can cover various skin colors and arterial elasticities, providing more accurate data for testing wearable devices.
- High reproducibility: Multiple tests are conducted under the same conditions, and the results are highly consistent, helping to shorten development time and reduce clinical trial costs.
- Multifunctional application: Apart from PPG testing, AECG100 can simultaneously output ECG and PPG signals, adjust the time difference between the two signals (PTTp, PTTf), verify the accuracy of the blood pressure measurement algorithm of the object to be measured, satisfying more kinds of testing needs.
Provides customized fixture and finger socket services to align the LEDs and PDs of the DUT and testing equipment for achieving more precise test results.