"UDiBar is user-friendly, effectively saving time and training costs for UDI implementation."
-  Winnoz Technology
Winnoz Technology
UDI, which stands for Unique Device Identification, is the foundation for precise identification of medical devices. Following internationally recognized coding standards, it consists of a Device Identifier (DI) and a Production Identifier (PI) made up of numbers and letters to describe information about a medical device. It's essentially the social security number for medical devices, with each set being uniquely tied to a single device.
The use of UDI for tracking medical devices is a global consensus. While the timing of UDI implementation may vary due to regional regulations and the class of medical devices, for manufacturers aiming to enter foreign markets, adopting UDI is indispensable.
However, implementing UDI is not as straightforward as one might think. After applying for a company prefix, manufacturers must maintain a database of UDI codes, redesign equipment labels and product packaging to comply with UDI requirements, and adjust production processes to meet UDI standards. In addition, it's essential to manage the UDI database with the necessary systems and processes and conform with relevant regulations to continually monitor and assess UDI implementation.
In the face of these challenges, Winnoz rapidly achieved compliance with UDI regulations and efficiently entered multiple foreign markets by adopting WhaleTeq's UDiBar.
The Company
Winnoz is a biotechnology research and development company dedicated to advancing "decentralized healthcare" and "biosensing" technology. Their vision is to promote the widespread adoption of preventive screening through point-of-care testing (POCT) for blood collection and testing, enabling individuals to undergo testing from anywhere. This, in turn, offers customers more efficient and cost-effective health management services. By doing so, they aim to make medical resources more accessible and contribute more to human health management.
Winnoz's Haiim Blood Collection System is currently the world's only vacuum-assisted device for blood microsampling at fingertips, designed to assist health professionals in collecting a sufficient and high-quality blood sample while avoiding the generation of biowaste, facilitating necessary subsequent testing.
In addition, their eGGi Molecular Detection System employing isothermal amplification technology is compatible with most commercial assays and offers customized services based on customer requirements. It has a wide range of applications in the fields of healthcare, environment, food safety, agriculture, and animal husbandry for real-time testing.
The Challenges
To comply with UDI regulations, Winnoz encountered the following challenges and requirements after gathering information and evaluating their current status:
| Challenges | ||
| UDI Code and Label Management | Interdepartmental Workflow and Integration | |
| Requirements | • Familiarize with UDI product and packaging level coding rules and establish a management system for archiving production information after creating PI codes each time • Store original labels and printing records of products and packaging for future reference • Comply with ISO 13485 management standards to effectively control product quality | • Define permissions based on departmental responsibilities and create a process for confirming UDI label information • Use a single system or platform to manage UDI data, synchronizing information across departments • Directly share UDI data with label editing software to avoid errors from manually copying information |
The Solution
After evaluating options, Winnoz preferred to implement UDI through an integrated system. WhaleTeq's "UDiBar - UDI Generator and Label Management System" providing one-stop UDI management met their requirements in two key ways:
• Fully compliant with UDI regulatory requirements
• Supports file formats for uploading to each country's database
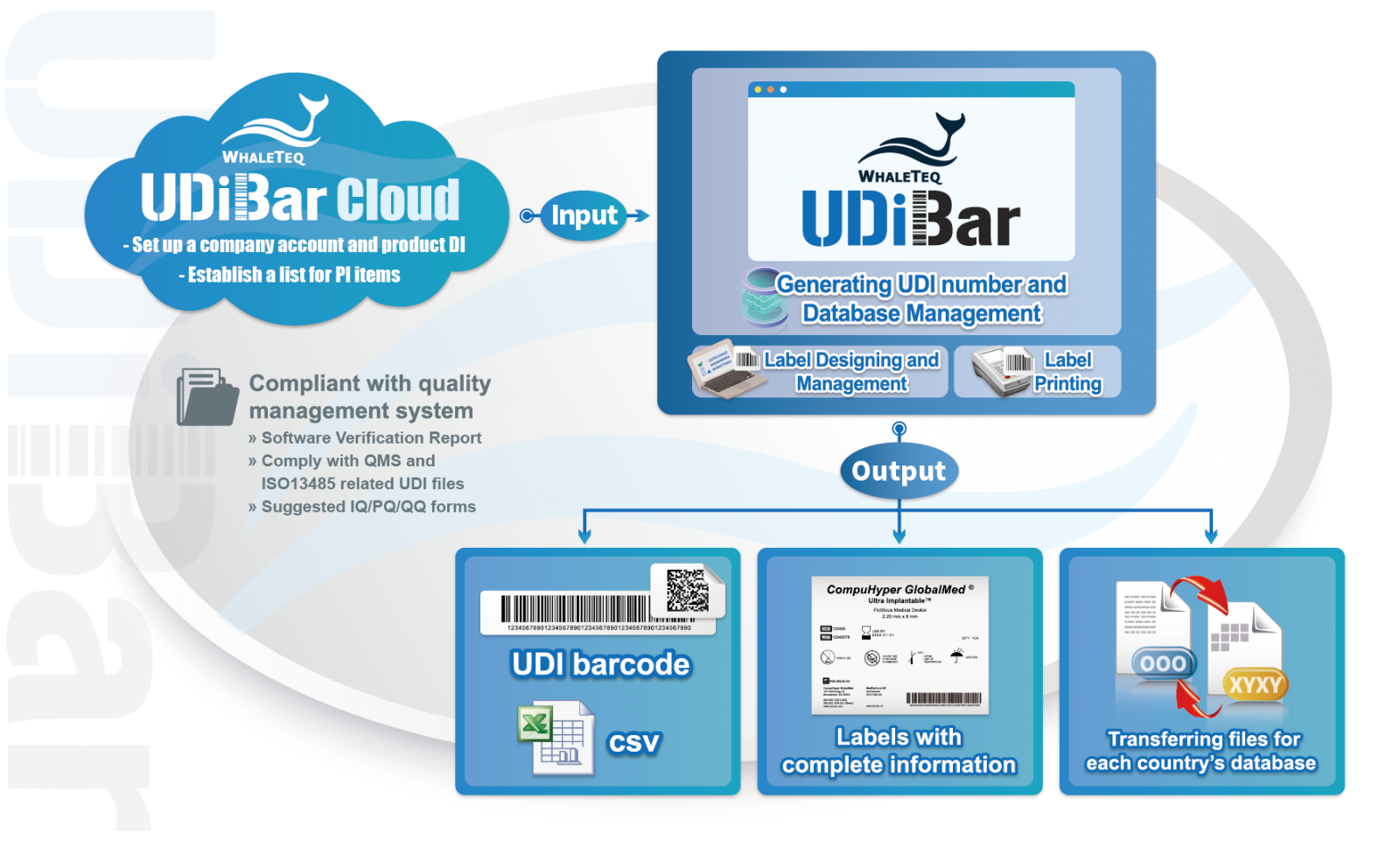
The Benefits
| UDI Code and Label Management | Interdepartmental Workflow and Integration |
| • With UDiBar's built-in steps and templates, Winnoz effortlessly complies with regulatory requirements for packaging coding and can automatically store production information • Through the UDiBar's user interface, they directly edit labels, including adding symbols complying with ISO standards and generating one-dimensional and two-dimensional codes, and track label printing records • WhaleTeq provides the software validation report for UDiBar, along with UDI-related 2nd, 3rd, and 4th-level document templates required by QMS/ISO 13485, to meet international quality management system standards | • By defining administrator and user permissions based on departmental responsibilities in regulation, quality control, and manufacturing divisions, Winnoz ensures that only information reviewed and approved by administrators can be used, thus guaranteeing accuracy • All UDI data are stored in the UDiBar database, eliminating the need to switch between systems for searches • After review, UDI data are directly imported into the UDiBar software for label editing, eliminating the need for manual copying and pasting |
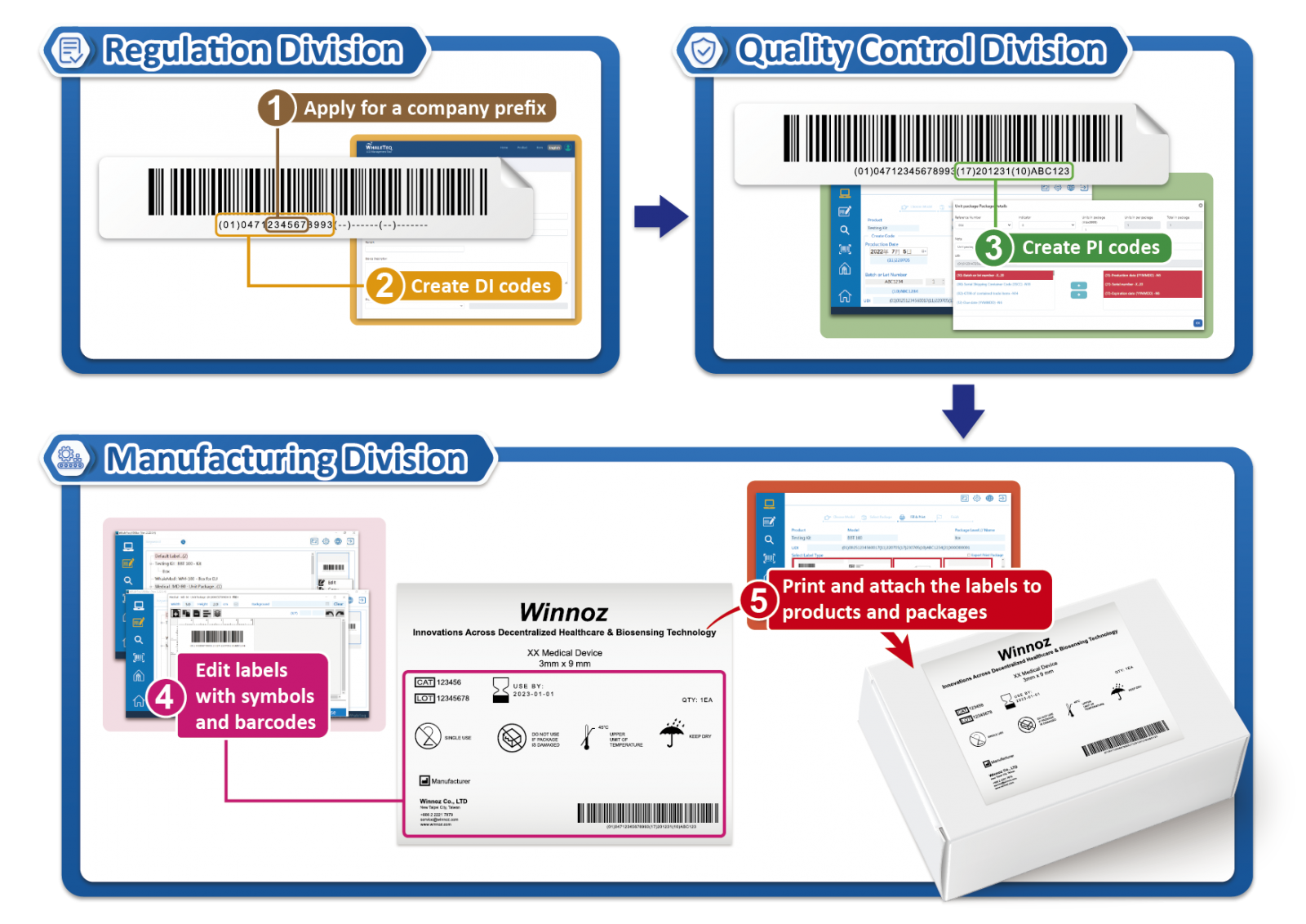
(Note: The barcode and label are for illustration purposes only and do not represent actual product information.)
Before medical devices can be brought to market, they go through a lengthy process encompassing research, validation, and manufacturing. To assist manufacturers in meeting regulatory requirements and simplifying administrative processes, WhaleTeq developed UDiBar, aiming to integrate interdepartmental management involving multiple files into a single system.
Each department only needs to learn how to use one software and define permissions based on responsibilities. Any information changes are recorded in the database for easy management and retrieval. The "UDiBar - UDI Generator and Label Management System" can help manufacturers efficiently implement UDI, saving time and costs while reducing human error, and making medical device tracking easier.
Note: UDiBar's functions are continually being updated. Please visit its product page for the latest operating instructions, user interface, and other information.
• Product Information: UDiBar
• Related Articles: 3 Reasons Why You Should Import UDI, How to Optimize the Introduction Process of the Unique Device Identification System (UDI)?