
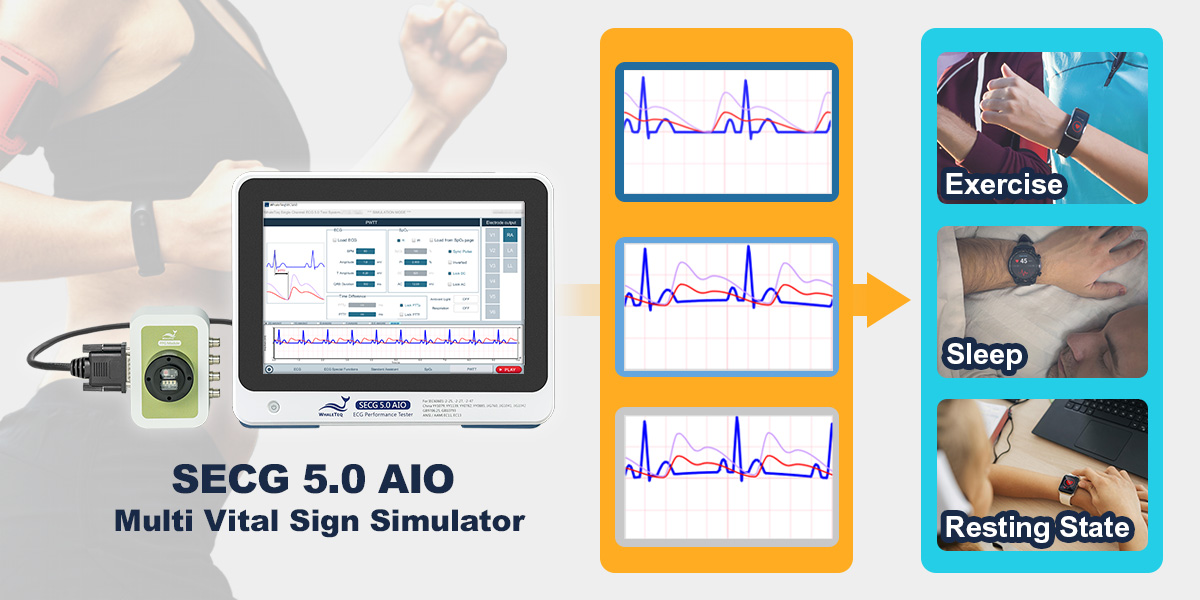
Monitoring physiological parameters through smartwatches has become a part of everyday life. In response to user demands, technology companies have taken a step further by developing smart rings, minimizing discomfort while maintaining high-quality measurement performance. Whether it's smartwatches or smart rings, manufacturers face challenges related to integrating technologies, verifying designs, and ensuring high accuracy and reliability. Addressing these development needs, WhaleTeq is pleased to announce the evolution of the SECG 5.0 AIO Multi Vital Signal Simulator, now compatible with the PPG-2R-880, PPG-2R-940 reflectance SpO2 module, and the PPG-1R-525 reflectance green light heart rate module, enabling PWTT (Pulse Wave Transit Time) or SpO2 testing.
Synchronizes PPG and ECG Signals for PWTT Testing to Verify Blood Pressure Measurement Algorithms
The PPG-2R-880, PPG-2R-940, and PPG-1R-525 employ PPG technology and, when used with the SECG 5.0 AIO, allow for synchronizing ECG and PPG signals for PWTT or PWV (Pulse Wave Velocity) testing. Developers can adjust the time difference of PWTT to simulate different measurement scenarios formed by vascular conditions, thus testing the accuracy of non-invasive blood pressure (NIBP) measurement algorithms in wearable devices. The SECG 5.0 AIO also provides respiratory simulation parameters superimposed onto PWTT signals to simulate real-life situations such as sleep, exercise, and other states, ensuring the interpretation capabilities of products.
(The image is for illustration purposes only and does not represent waveforms of real-life situations.)
Builds SpO2 R Curves for Rapidly Verifying the Consistency of Product Performance
For SpO2 testing, developers can utilize the PPG-2R-880 and PPG-2R-940 modules to finely adjust the AC, DC, and PI parameters of red and infrared light in the software interface to test SpO2 measurement algorithms. Using the values obtained from these tests, R curves for the product can be generated to quickly verify if the performance of other devices of the same model is consistent and reliable. Additionally, this feature can be used to create R curves of competitive products to compare different measurement designs.
As the variety of health monitoring devices continues to expand to meet market demands and technological advances, effectively enhancing verification efficiency and accuracy during development is key to meeting market expectations at an accelerated pace. WhaleTeq believes that test equipment must move with the times to meet manufacturers' research and development requirements, thus contributing to the creation of higher-quality medical devices.
For more information, please visit the product page: SECG 5.0 AIO