News Source: TechNews

To help people get out of the quagmire of reactively receiving medical treatment, proactive preventive medicine has become a new science with great development potential. A wearable device has become the best tool for monitoring vital signs anytime and anywhere.
Since 2013, WhaleTeq has been committed to the ultimate vision of making all medical and wearable devices more accurate, thereby accelerating the development of decentralized medical systems and preventive medicine.
Find the Correlation between Vital Signs and Potential Diseases through Precise Medical Devices
Nathan Lin, CEO of WhaleTeq, strongly advocates that only by changing the current medical system can we create a "four-win" situation for patients, hospitals, insurance companies and the government. The best practice is to change from a hospital-centered architecture of the past to a decentralized medical system centered on individuals and family.
To become aware of potential diseases, we must find their relationship with vital signs, including ECG, body temperature, weight, height, blood pressure, blood sugar, respiration, blood oxygen, etc. The premise of all the above is the need for monitoring equipment of high quality available at home.
The four major technology giants, Apple, Huawei, Google and Samsung, are optimistic about the potential of the decentralized medical market. They have already launched various wearable devices that support vital sign monitoring functions. These manufacturers have achieved convenience and portability that were difficult to achieve with medical device in the past. Without these features, daily monitoring with ease is impossible.
The current state of the market is "All is ready except for the opportunity.” This "opportunity" refers to precision. Faced with the goal that all medical device manufacturers are unanimously pursuing, WhaleTeq hopes to play an "Enabler role".
Assist 900 Customers around the World to Easily Improve the Accuracy of Medical Devices
WhaleTeq is the world's first supplier dedicated to providing test solutions for medical device manufacturers, especially for R&D departments. With the strong support of WhaleTeq, the accuracy and quality of medical devices have been significantly improved. However, even mature blood pressure monitors on the market may produce inconsistent measurement results. This has led doctors to rely only on the "relative" results of the measurement data, and patients with high blood pressure are forced to have more than one blood pressure monitor at home.
The primary method to ensure the accuracy of medical devices is through clinical trials. Large-scale clinical trial studies can optimize the algorithms of medical devices such as blood pressure monitors. Nevertheless, clinical trials are costly, time-consuming, and labor-intensive, making them unaffordable for most manufacturers. To minimize trial and error and reduce costs, medical device companies are eager to find solutions that can efficiently conduct performance and standard tests before clinical trials.
Nathan(CEO) assessed that even though there are test devices currently on the market, they are all low-end products used in hospital after-sales services and can’t meet the testing needs of manufacturers and laboratories. No matter what, the fundamental of medical devices lies in accuracy. How to effectively improve the measurement results of medical devices while striking a balance between time and money has ,for a long time, been the most common pain point for manufacturers .
First-Ever Blood Pressure Simulator Passed ISO/TS 81060-5 Standard
Nathan also pointed out that to make the data from blood pressure monitors more reliable, WhaleTeq released the BPA700 Non-invasive Blood Pressure Simulator last October after 5 years of development spanning 9 generations of experiments. This product can simulate various blood pressure conditions and waveform data observed in real clinical settings, thereby simplifying and reducing the cost of clinical trials. The device can also connect to blood pressure monitors to directly record and store patients' blood pressure values for future playback, facilitating validation and optimization of the monitors' algorithms.
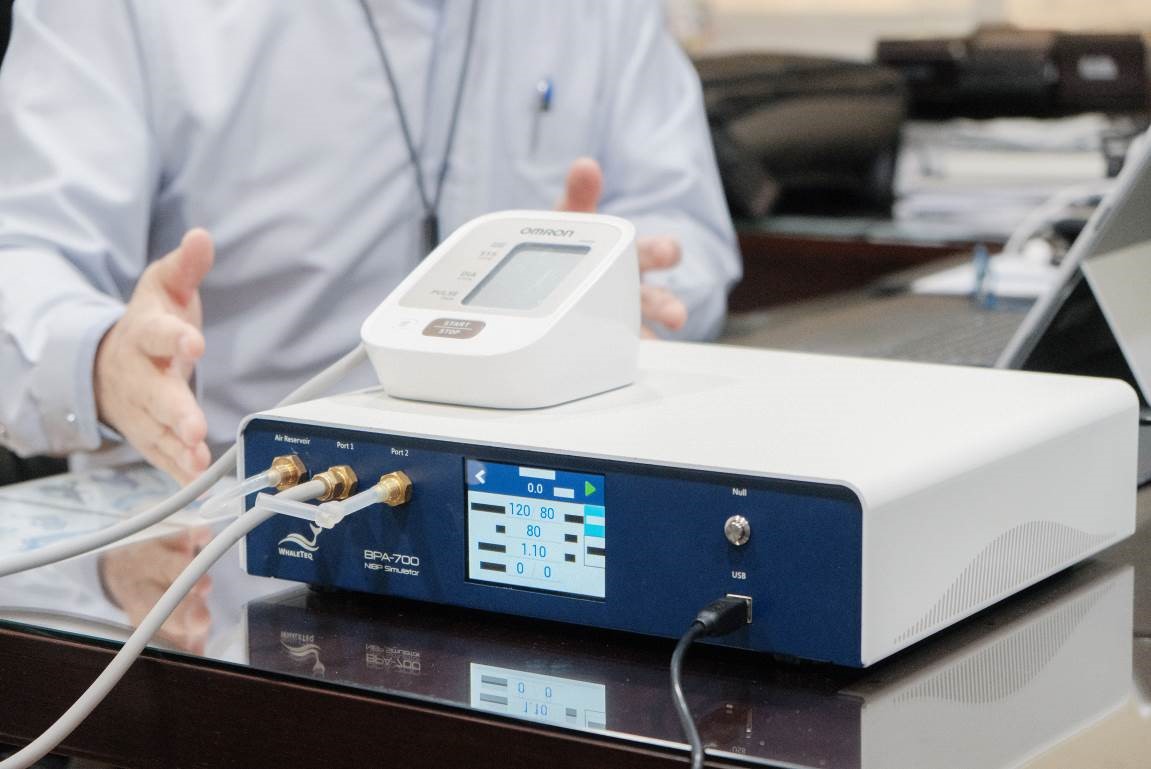
▲The BPA700 Non-invasive Blood Pressure Simulator can simulate various blood pressure conditions and waveform data of real clinical patients, which not only reduces the complexity of clinical trials, but also saves more costs. (Source: TechNews)
Because the BPA700 preserves actual clinical data, it can compare and verify the readings from blood pressure monitors connected to the simulator. This effectively assists algorithm engineers in ongoing verification work, thereby optimizing the accuracy of the blood pressure monitors. It also ensures that the measurement performance of the blood pressure monitors used by customers becomes increasingly close to reality.
Furthermore, since the BPA700 can store various types of blood pressure data, it can accurately simulate waveforms of special conditions such as arrhythmias, which greatly aids in diagnosing a wider range of conditions.
The BPA700 is compatible with all types of blood pressure monitors, including wrist, arm, and cuff types. It can be operated standalone or connected to a computer. The device is of high accuracy, capable of achieving control within one or two mmHg, and provides a wide range of simulation displays, with dynamic blood pressure up to 300 mmHg and static blood pressure up to 400 mmHg.
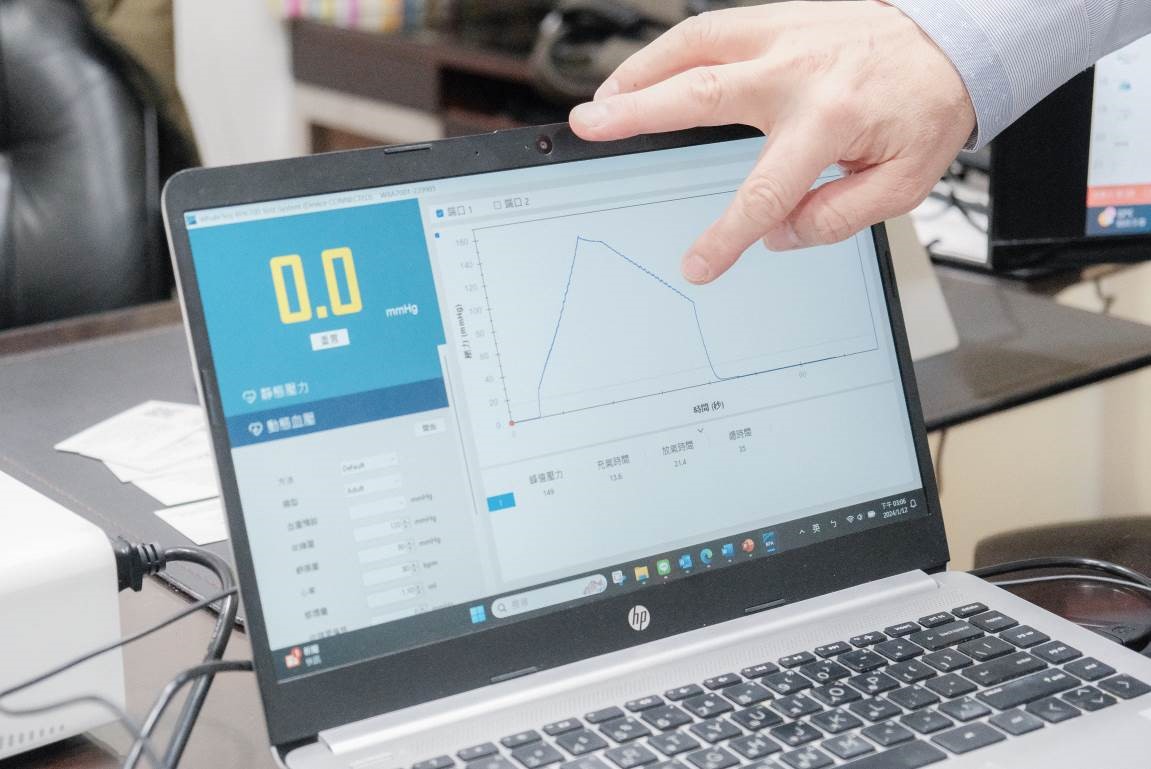
▲BPA700 is compatible with all types of blood pressure monitors, including wrist, arm, and cuff types. It can be operated standalone or connected to a computer. (Source: TechNews)
The product complies with the IEC 80601-2-30 Standard, and WhaleTeq has integrated the relevant test requirements and test clauses of this standard into both BPA700 and its exclusive software. Customers can easily complete the entire standard testing process by following clear and simple steps, significantly reducing the overall testing and verification time. This is a major selling point of the product.
The BPA700 also features an industry-first adjustable pulse envelope, allowing users to easily and conveniently simulate various blood pressure conditions of real clinical settings. The device also includes a waveform data playback feature, which will soon be released. Users can utilize this feature to play back data at any time, thereby validating and optimizing algorithms and product designs.

▲With the help of the industry's first "adjustable pulse envelope" function , it becomes more convenient for users to simulate various blood pressure conditions of real clinical settings. (Source: TechNews)
In addition, the BPA700 is not only the world's only high-end NIBP tester, but also the world's first ever blood pressure simulator to pass the ISO/TS 81060-5 new International Standard. This demonstrates that the device's ability to assist blood pressure monitors in becoming more accurate is recognized internationally.
WhaleTeq has achieved many remarkable accomplishments and innovations in the market, including the world's first to provide ECG International Standard testing solutions, wearable device testing solutions, AED after-sales maintenance solutions, and a UDI Generator and Label Management System. As a result, the company has gained the trust of customers, accumulating over 900 clients in 36 countries, including medical device manufacturers (R&D, QA, and production line), third-party laboratories, research institutions, and hospitals (such as medical engineering departments). Currently, almost all wearable device manufacturers are customers of WhaleTeq.
Furthermore, by combining its three major product lines of test devices, after-sales maintenance solutions, and regulatory software, WhaleTeq has launched a full lifecycle quality management service that meets the three principles of reliability, accuracy, and compliance required for medical devices. WhaleTeq has also designed specialized test devices for different objectives, including ECG, EEG, wearable devices, AED, NIBP blood pressure monitor, and patient monitor. These devices are complemented by Standard Assistant Software and algorithm analysis software.
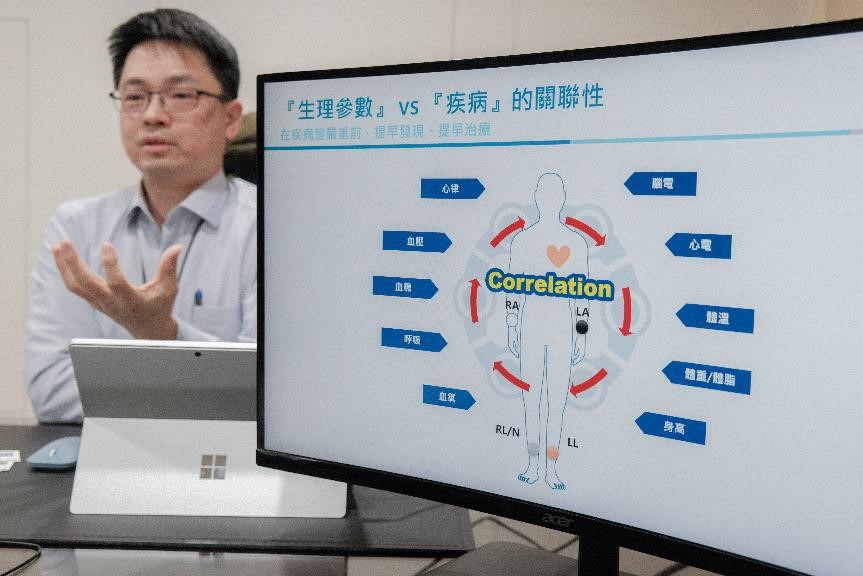
▲ There is a significant correlation between vital signs and diseases. WhaleTeq has designed a range of specialized test solutions, including ECG, EEG, wearable devices, AED, NIBP blood pressure monitors, and patient monitors. (Source: TechNews)
With the help of WhaleTeq's series of simple and easy-to-use test tools and solutions, manufacturers can save the time and cost of studying regulations and standards, designing test fixtures by themselves, etc., and then focus their resources on the innovation of their own products.
Under WhaleTeq's three major product lines, the company has been able to realize "four-win" situation with the entire medical ecosystem- patients, hospitals, insurance companies, and governments . This has also fulfilled the ultimate expectations of the public regarding healthcare: better medical care, affordable costs, and preventive medicine.