Origins
The identification and management of medical device is a very important part of controlling product flow and safety. WhaleTeq's test device meet the different testing needs of manufacturers in the R&D, production line and after-sales stages, while UDiBar- Medical Device Labeling Management Software can effectively control production identification information of medical devices before and after they are launched on the market.
From 2020, FDA has mandated that medical devices exported to the United States from all countries must introduce the Unique Device Identification (UDI) system; EU followed up in the same year and fully implemented the new regulations to strengthen the control of medical devices. At the same time, countries around the world have also successively announced the UDI introduction schedule.
The purpose of UDI is to improve the safety and traceability of medical devices within the supply chain, to more quickly identify affected products when recalls or adverse events occur, to investigate the causes of product problems and to monitor product performance and safety.
In response to global medical device manufacturers' needs for UDI label production, printing, and UDI management, UDiBar can assist medical device manufacturers in completing UDI compliance label production, printing, and UDI management in the most convenient way. It can also meet the traceability requirements of UDI and ISO13485 quality management system. In addition, to reduce the burden on regulatory personnel, UDiBar also has the advantage of updating regulations at any time to avoid misjudgments caused by human errors.
Features
Highlights
Compliant with the Requirements of ISO13485

- Comply with traceability requirement of ISO13485, which distinguish between different levels of user access and keep record of user activities
- Built-in medical icons that comply with ISO standards and can be used in UDI label editing
- Provide ISO13485 quality system related documents, including software validation reports, second, third and fourth level related documents and IQ/OQ/PQ forms
Allow Multiple Connections to UDiBar Database for Team Collaboration
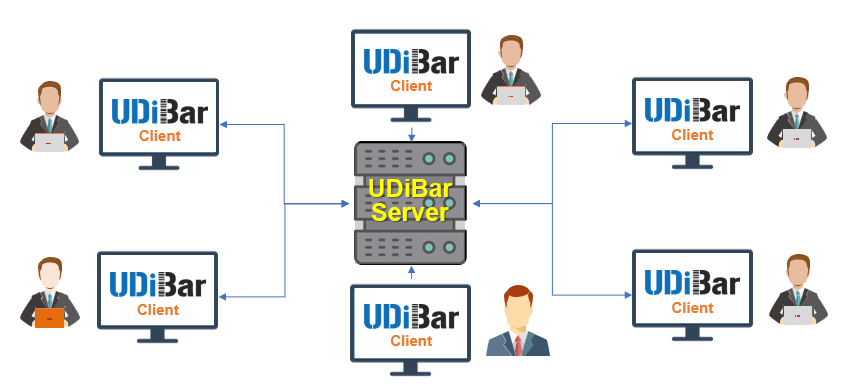
- Single authorization, no upper limit on the number of accounts, multiple computers in the same area network can work together to improve work efficiency
Able to Convert and Export EXCEL file for Each Format of Multi-national Database
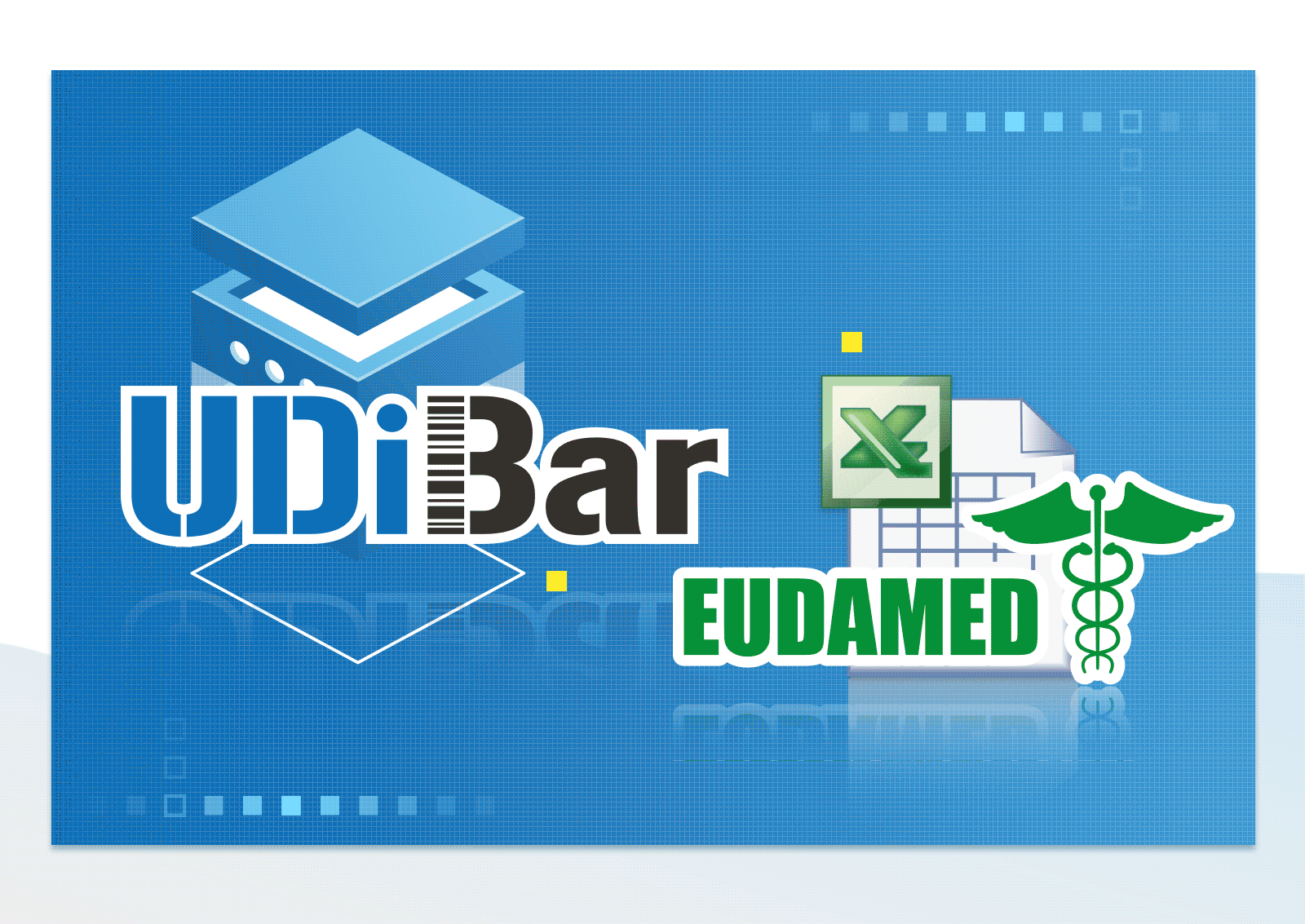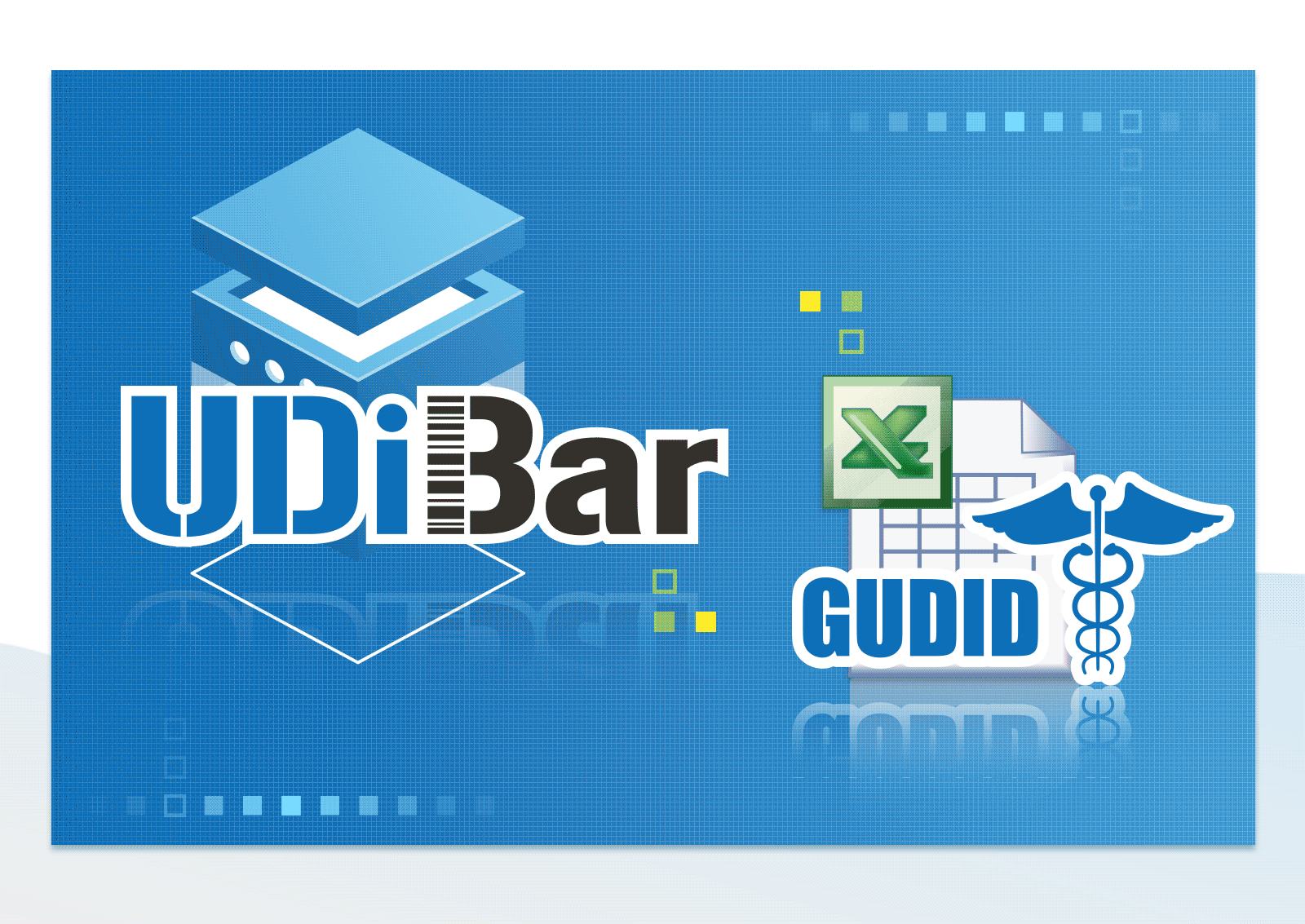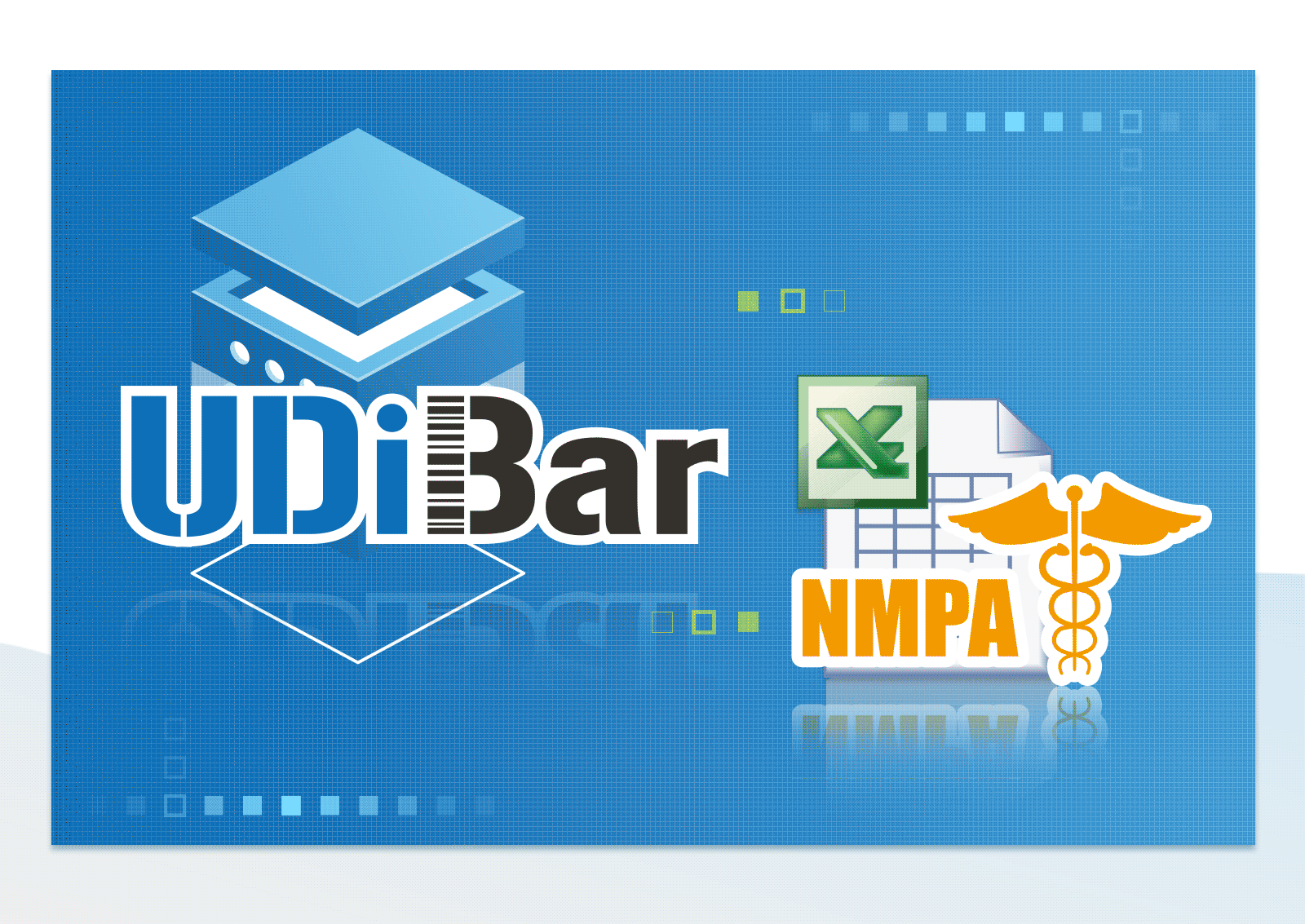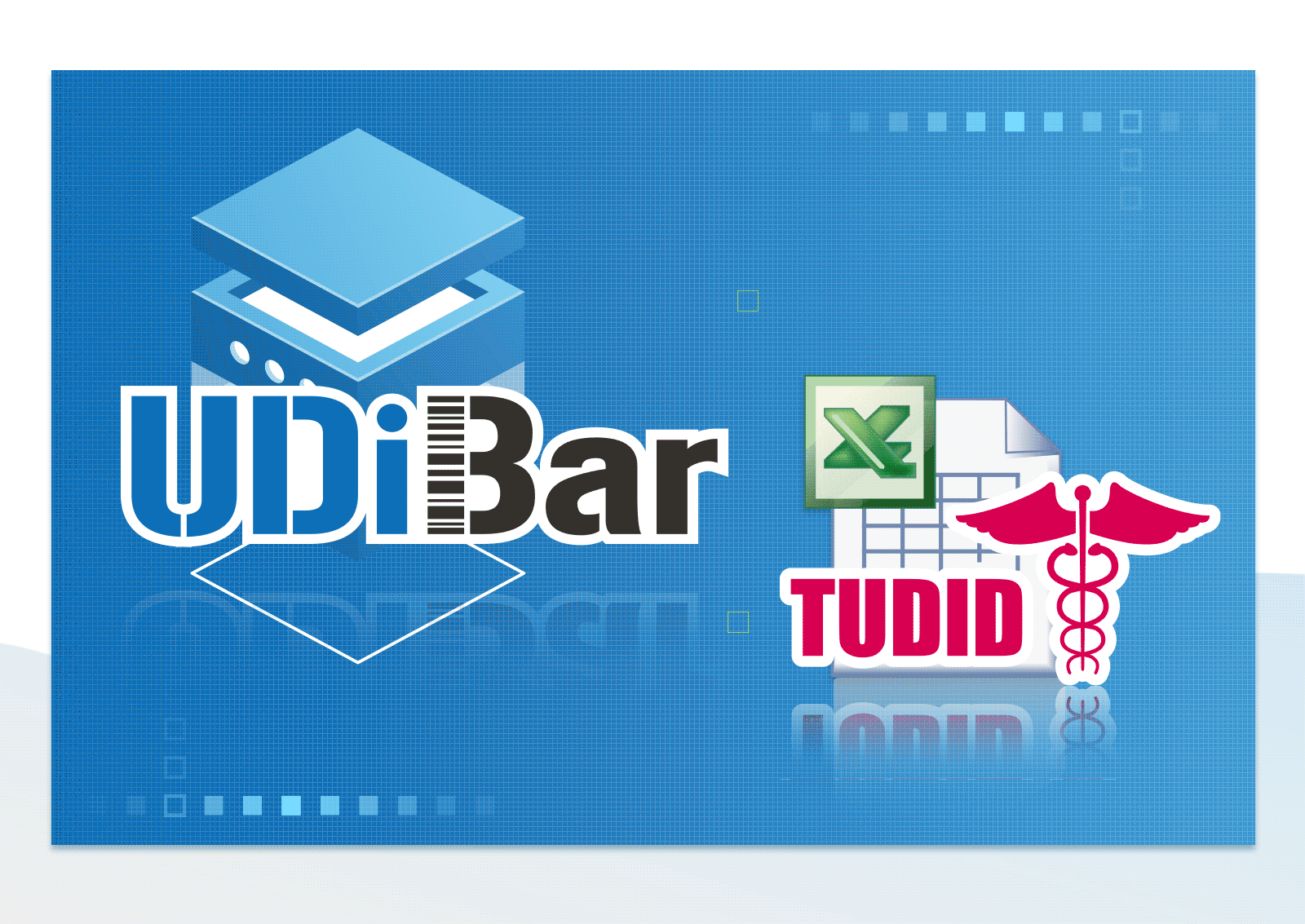
- Export EUDAMED/GUDID/NMPA/TUDID medical device database format EXCEL files
Link with External Management Systems like ERP, MES, or PLM

- UDiBar database can export CSV format files and connect to external systems
Specification
| Items | Specifications | |
|---|---|---|
| Language Support | English, Tranditional Chinese, Simplified Chinese | |
| User Account |
| |
| User Account Types | Administrator, User, Super User | |
| Multiple Connection | Multiple connections are allowed within LAN (Local Area Network) | |
| Supported Printer Driver | Argox, DOMINO, Zebra, Windows | |
| Support Barcodes | GS1-128, GS1-Data Matrix, EAN-13, Code-128, UPC-12, ITF-14, GS1 Databar, QR Code | |
|
UDI Database Conversion |
| |
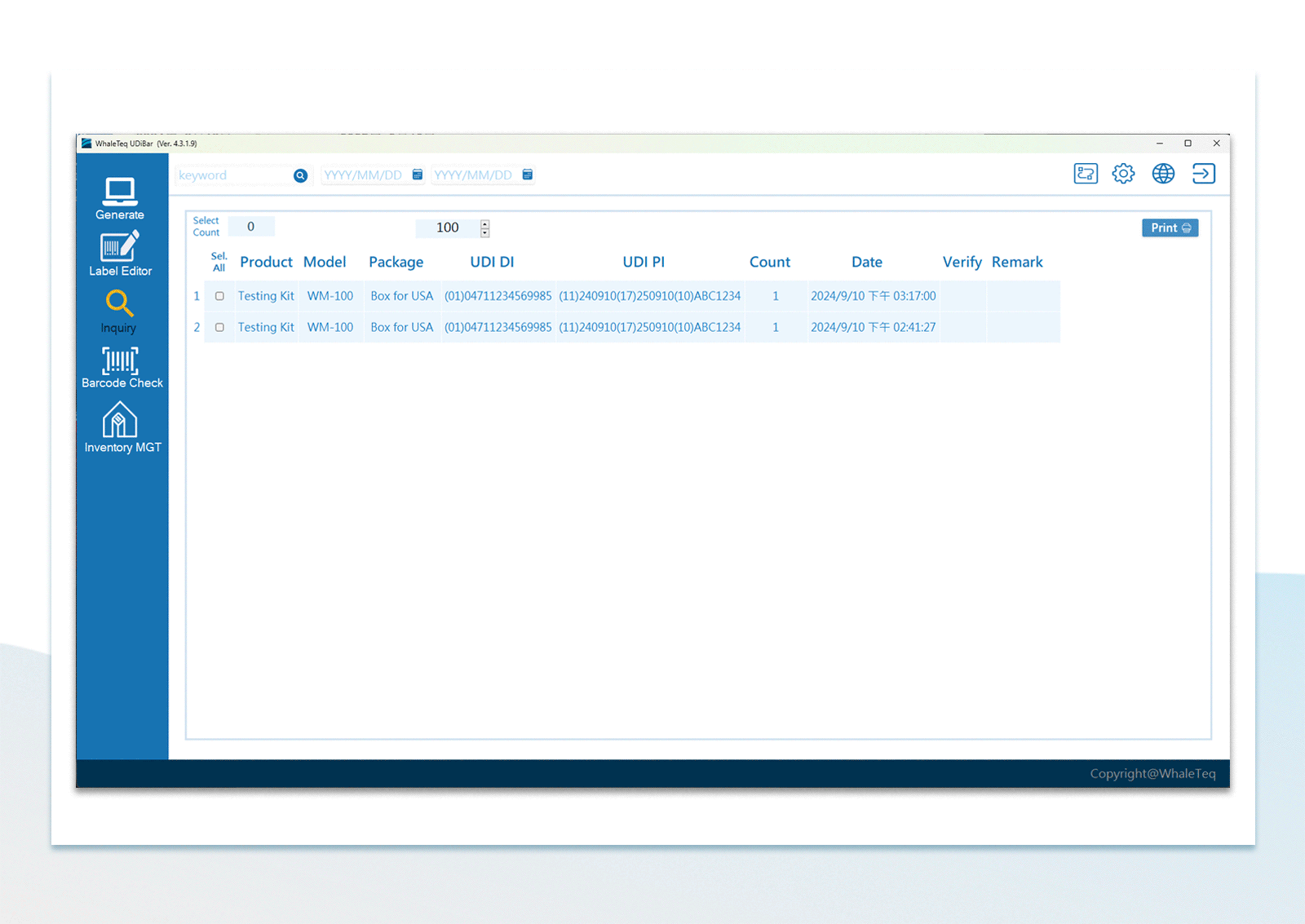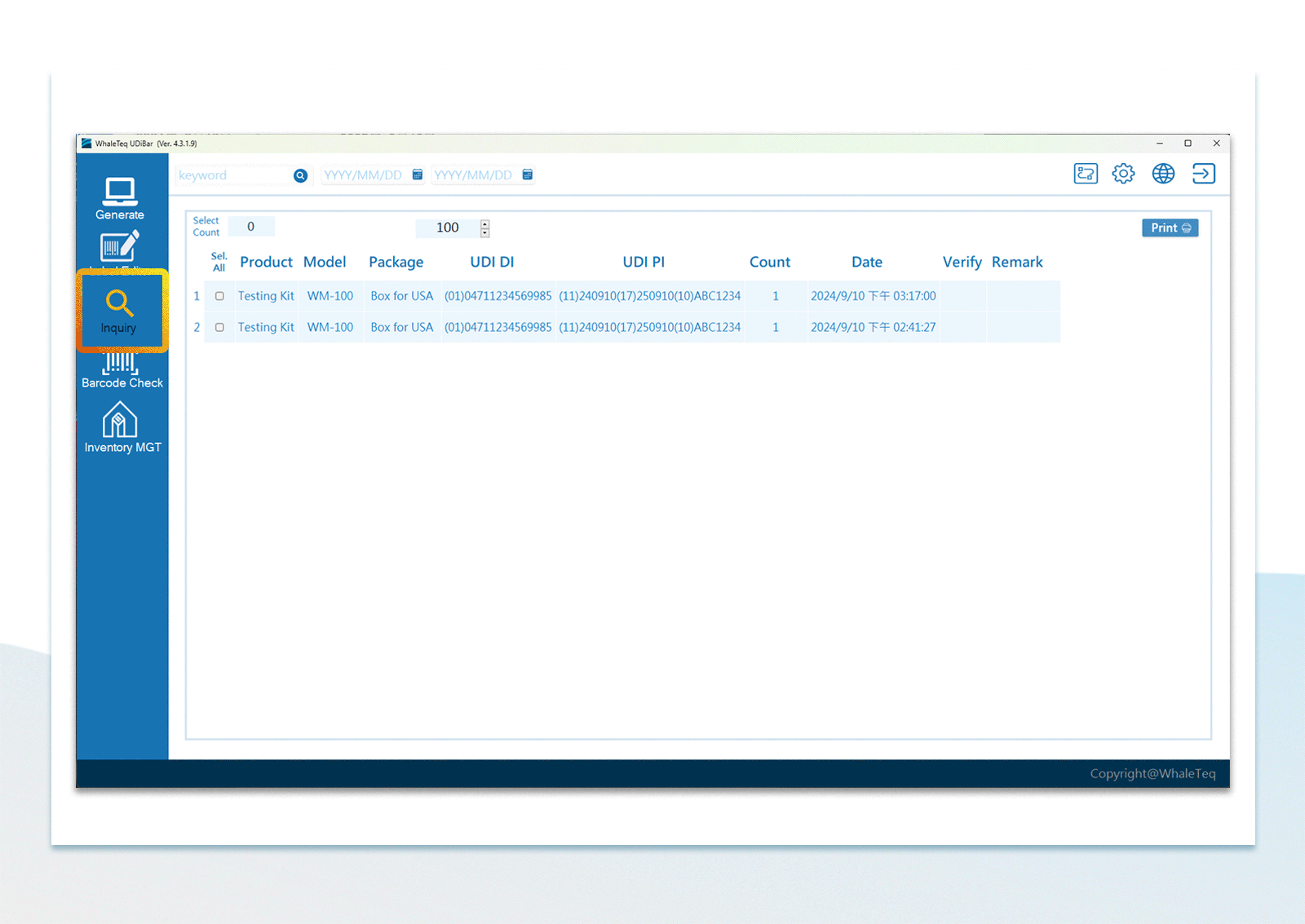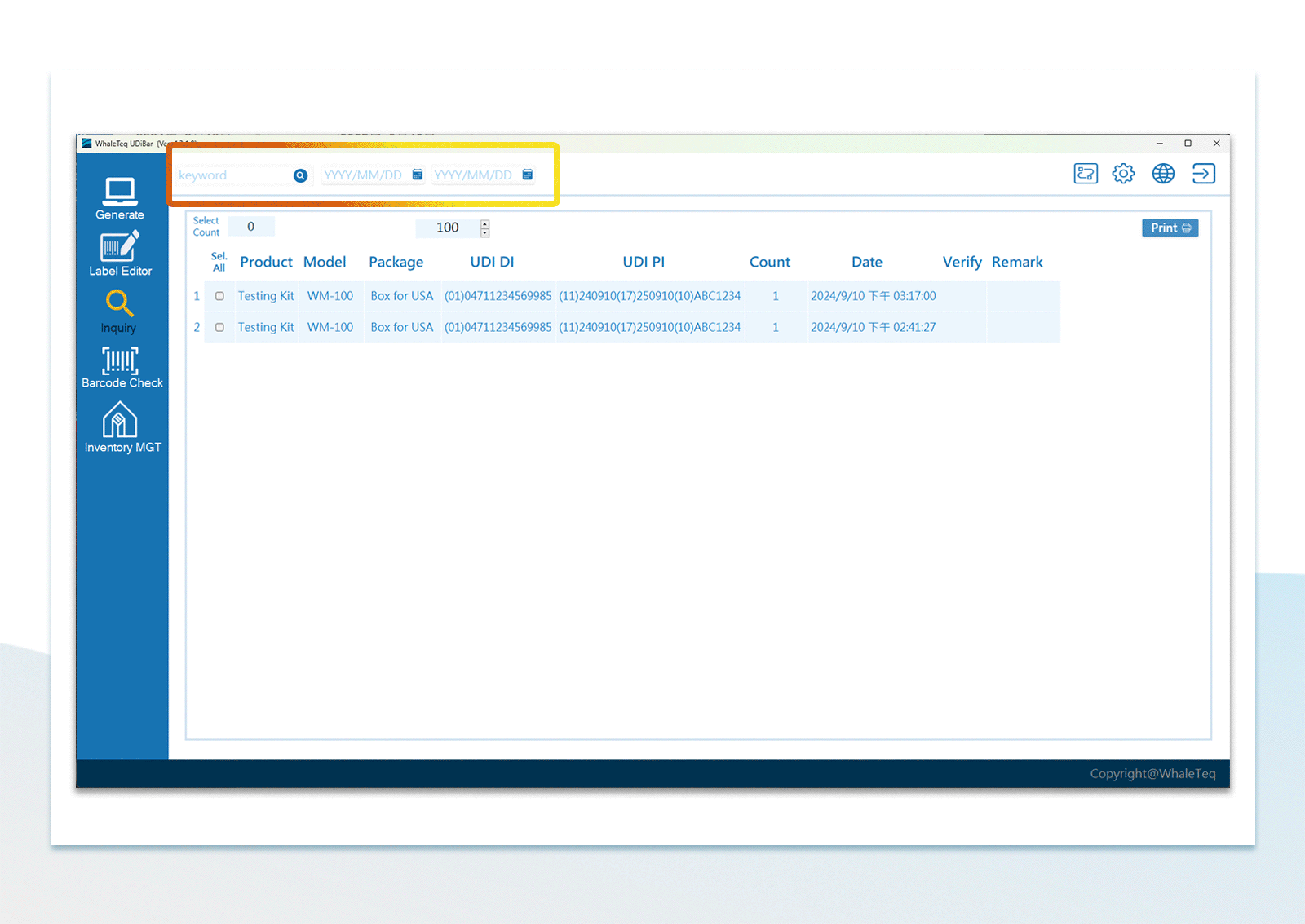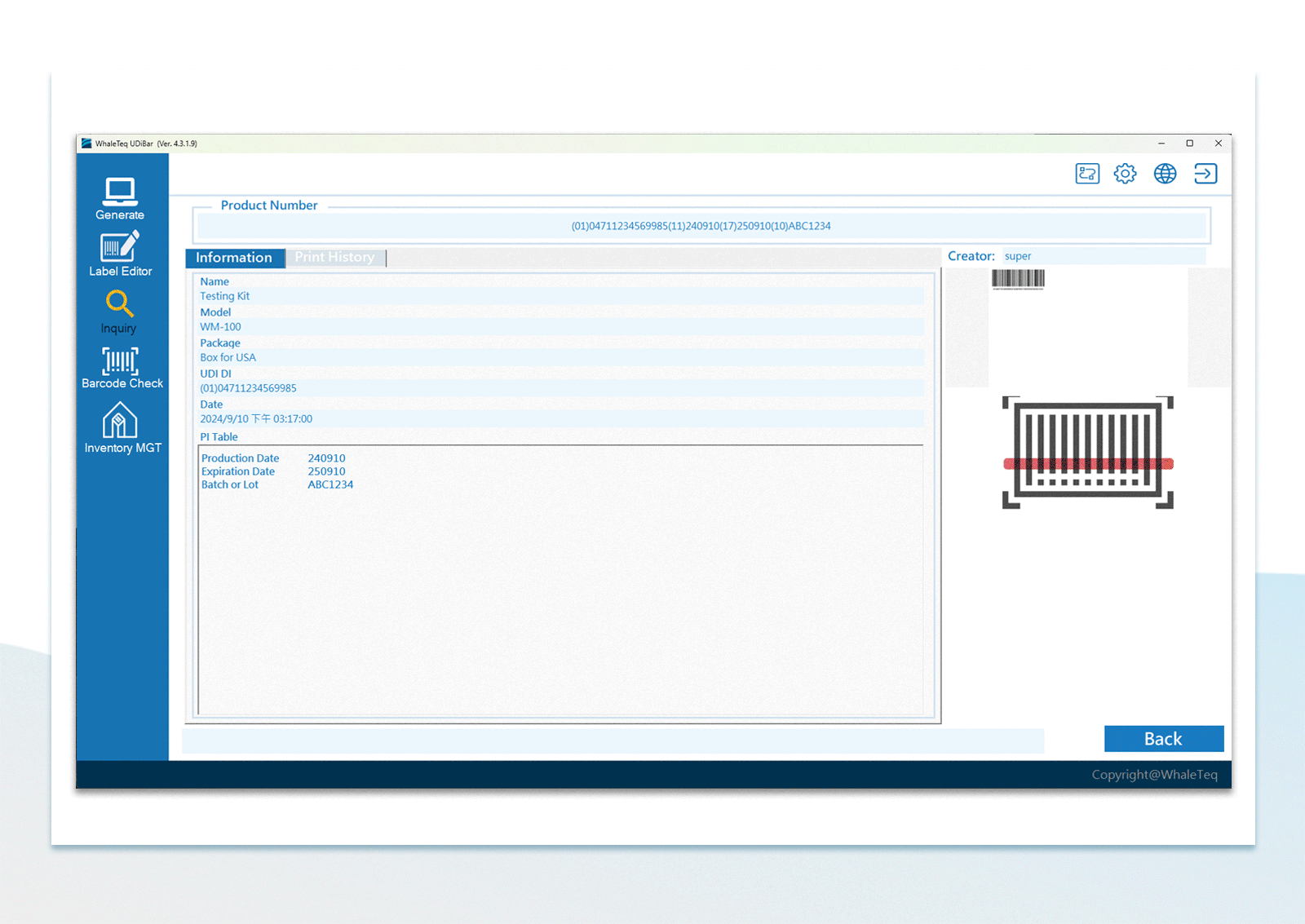
| Printed Label Check | Via the barcode scanner. Able to check the 1st label, the last label or assigned interval label | |
| UDI Database Inquiry | Keyword or the assigned time period | |
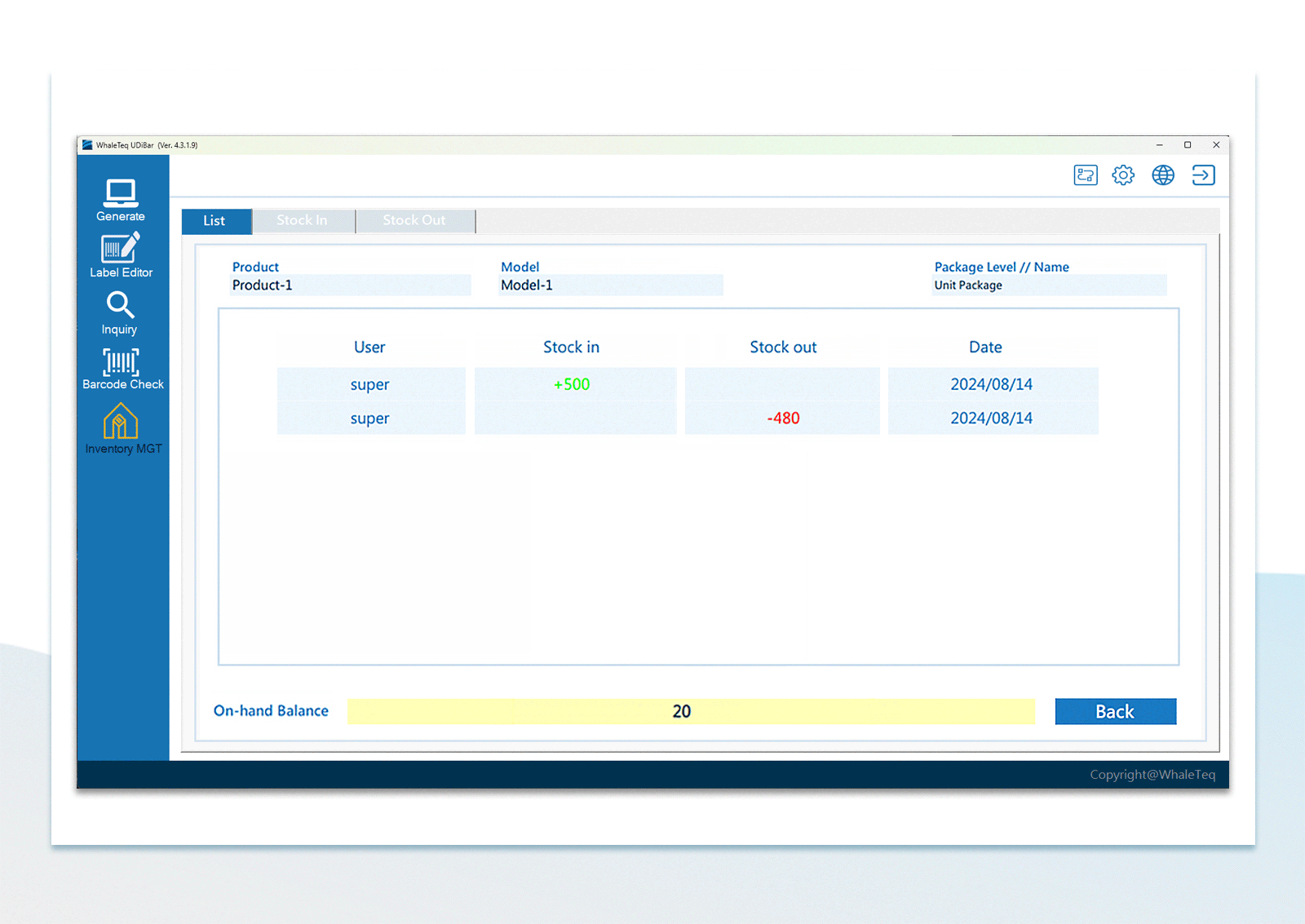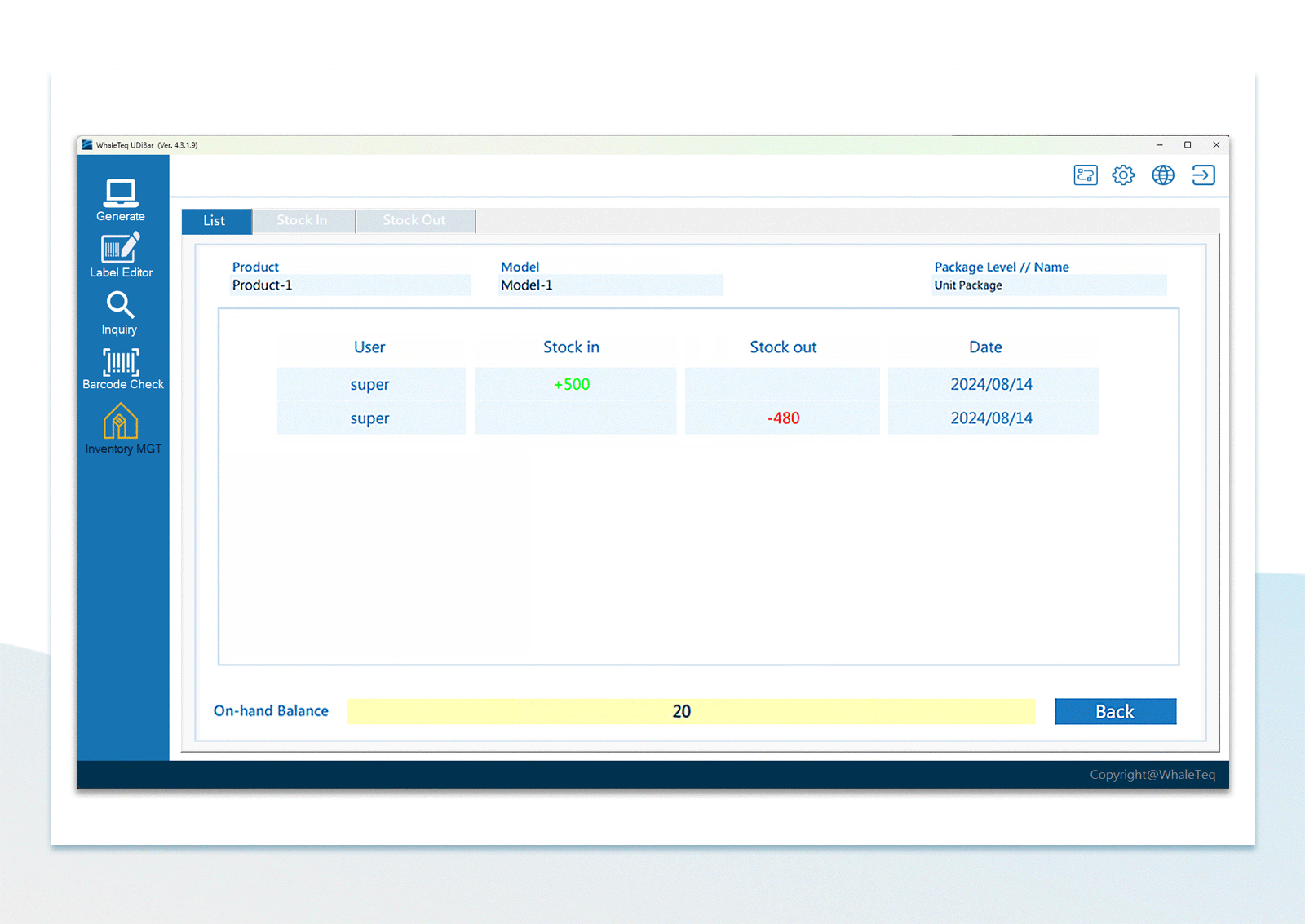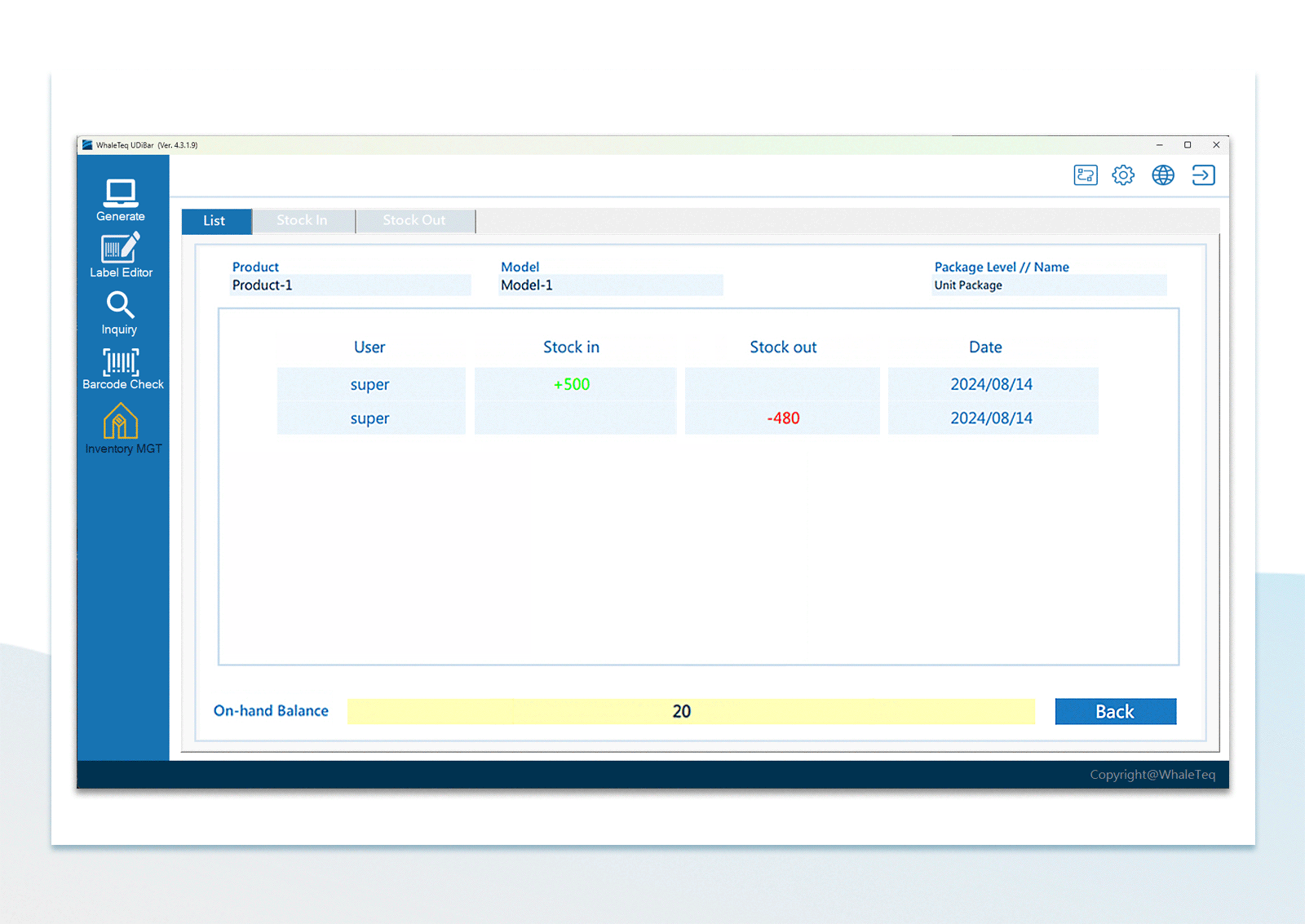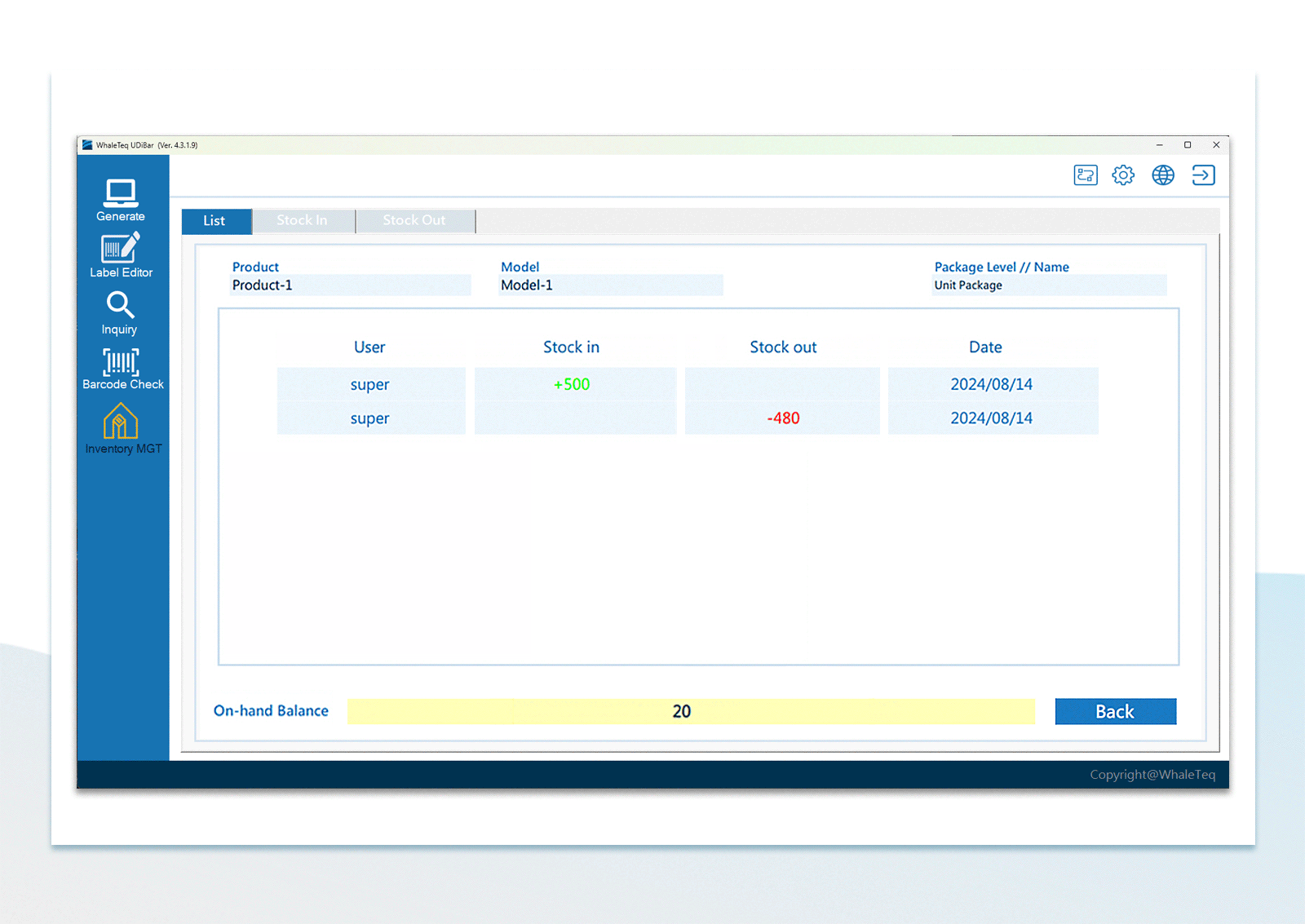
| Inventory Management | Use the barcode scanner to confirm in-stock items and out-stock items | |
| Import and Export Product UDI Information | CSV format file | |
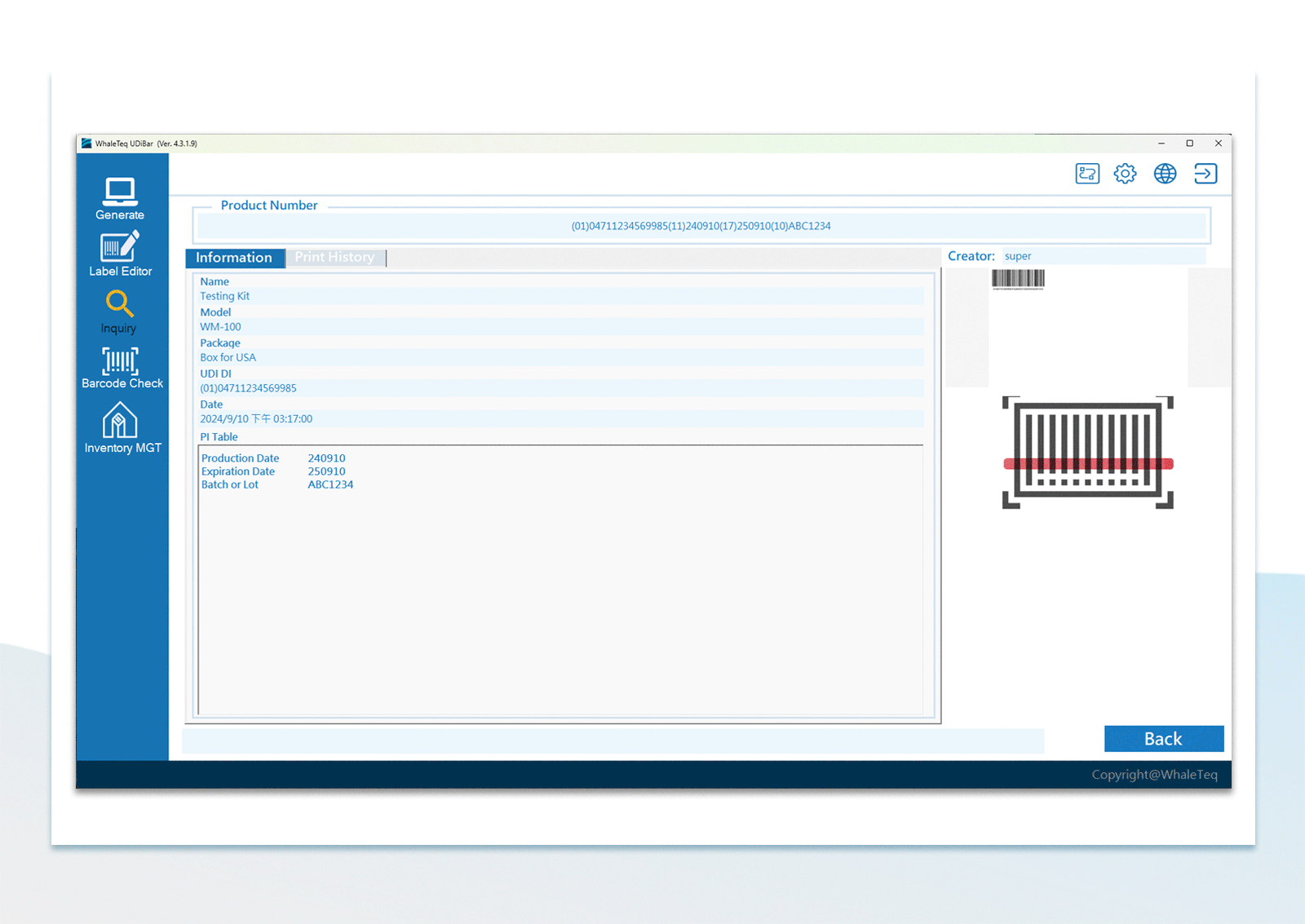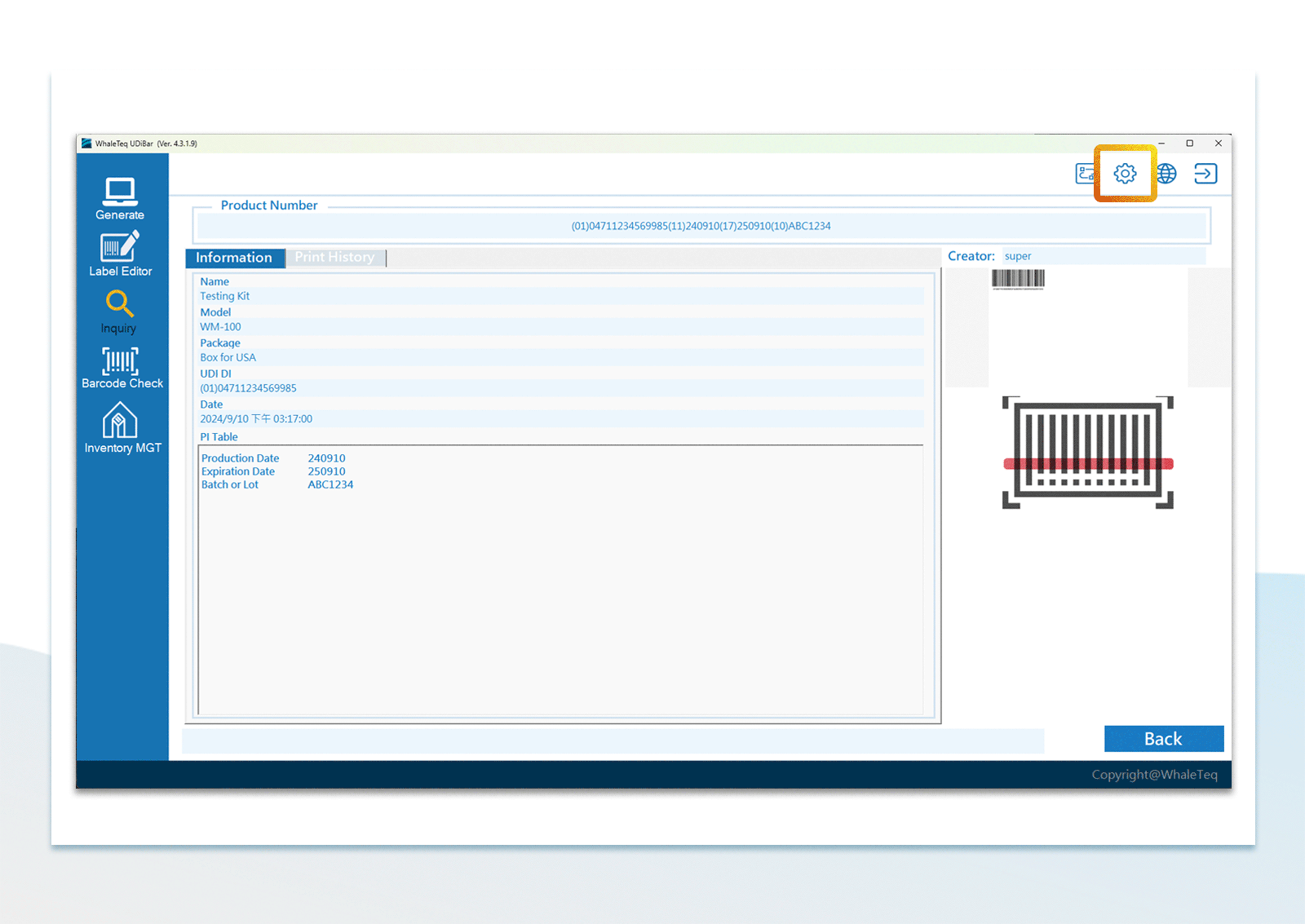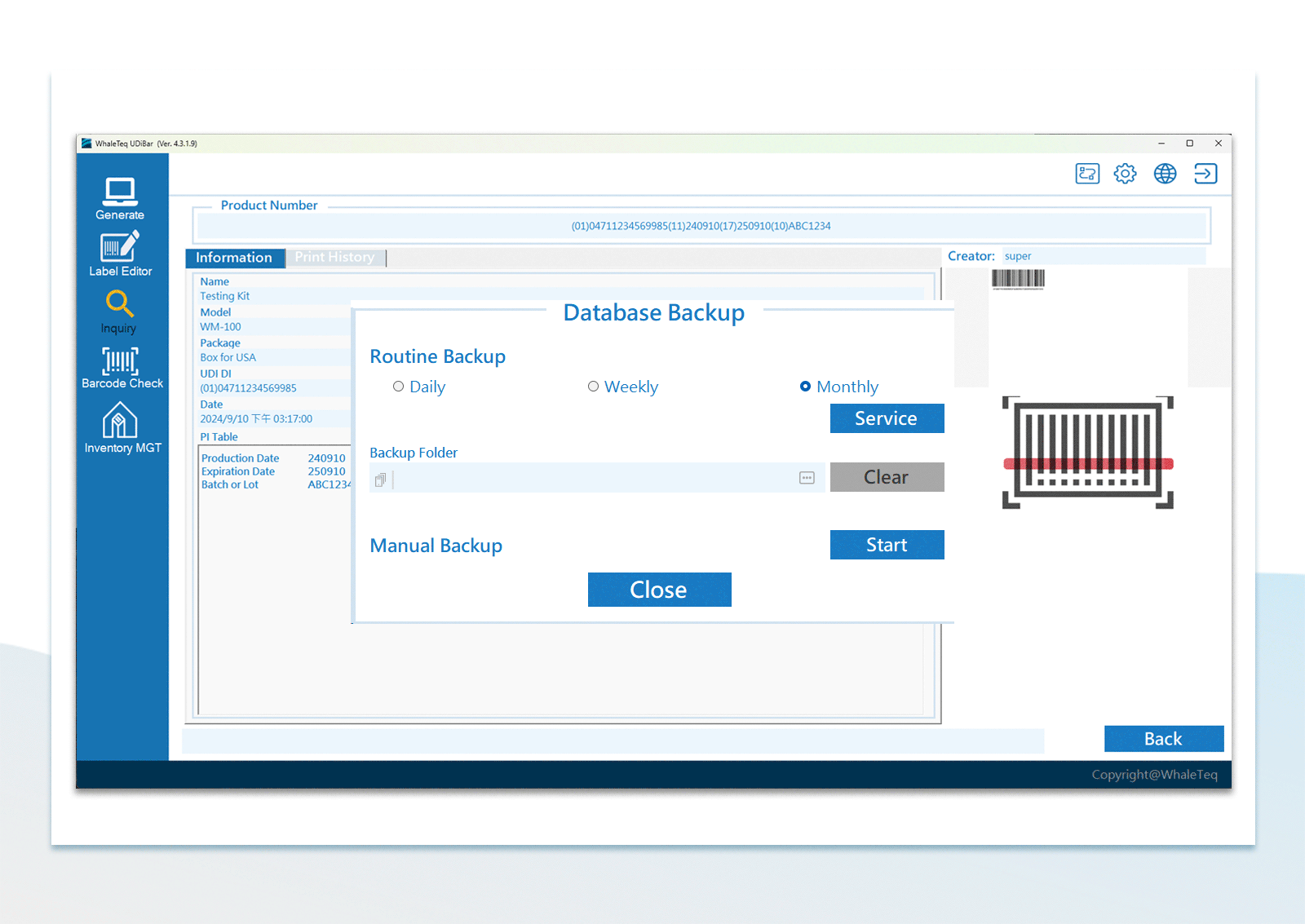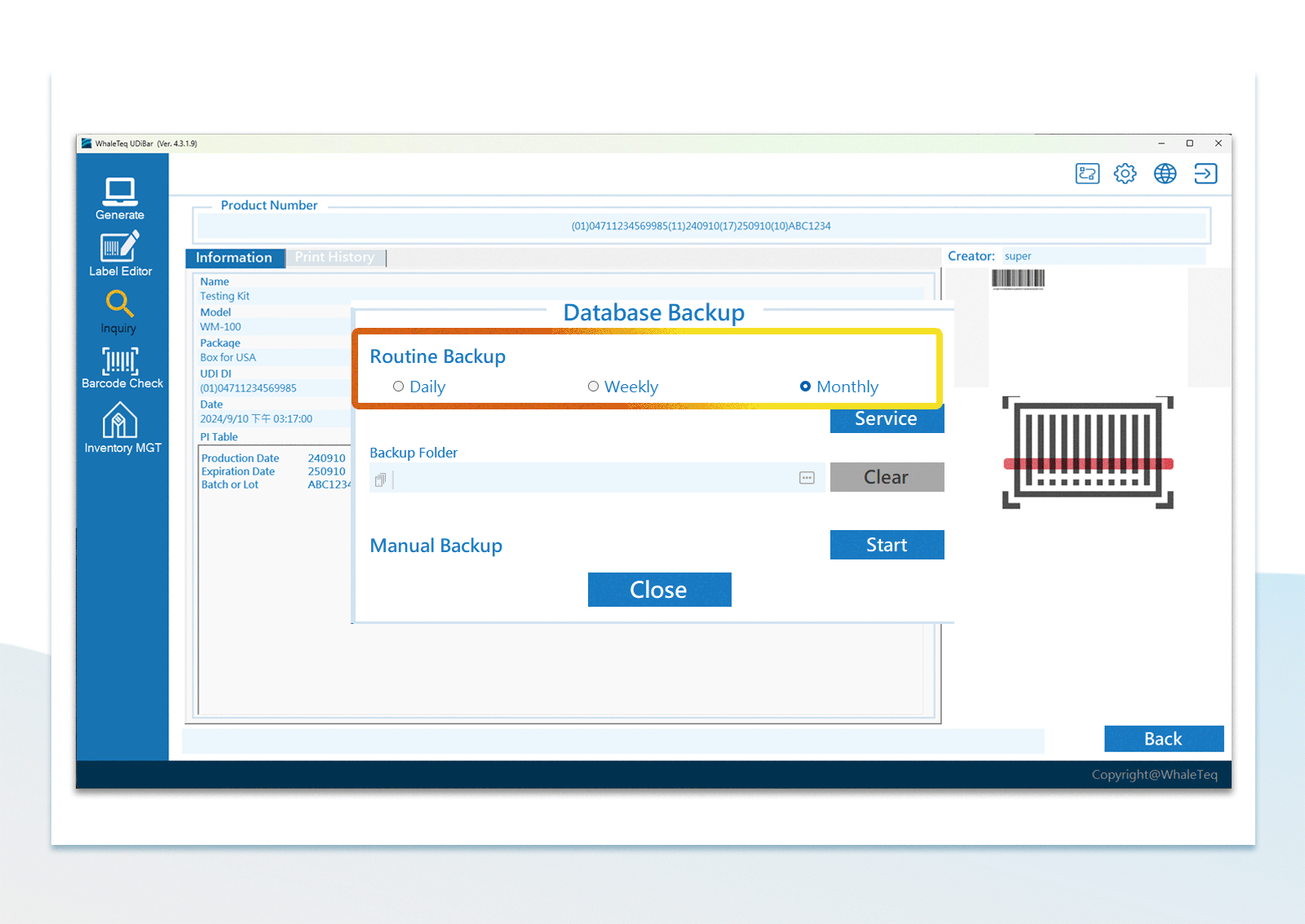
| UDI Database Backup | Manual backup or periodical backup within PC or other PCs in the same local area network | |
| ERP Integration | via CSV format file | |
Requirements
| System Requirements | |
|---|---|
| Items | Requirements |
| Operation System | Windows 10 64-bit or above |
| CPU | 1.5 GHz CPU or above |
| Memory | 4GB RAM or above |
| Hard Drive | At least 10GB |
| Internet Connection | Required when checking user licenses |
| Software Requirements | |
|---|---|
| Items | Specifications |
| Microsoft .Net Framework | 4.5 or Above |
| MySQL Community | 8.0 (Customzied MySQL for UDiBar software) |
| Microsoft Office | 2016 or Above. For editing the database conversion file |
Medical Device Labelling Management Software
| Items | Part Number | Description |
|---|---|---|
| UDiBar V4 | H40-UD00025 |
|
| UDiBar V4 Consulting Service | H40-UD00026 |
|
| S1 Extended Warranty | H40-UD00024 | UDiBar software license and software updates for additional (1) year. |
| S2 Extended Warranty | H40-UD00017 | UDiBar software license and software updates for additional (2) year. |
| S4 Extended Warranty | H40-UD00018 | UDiBar software license and software updates for additional (4) year. |






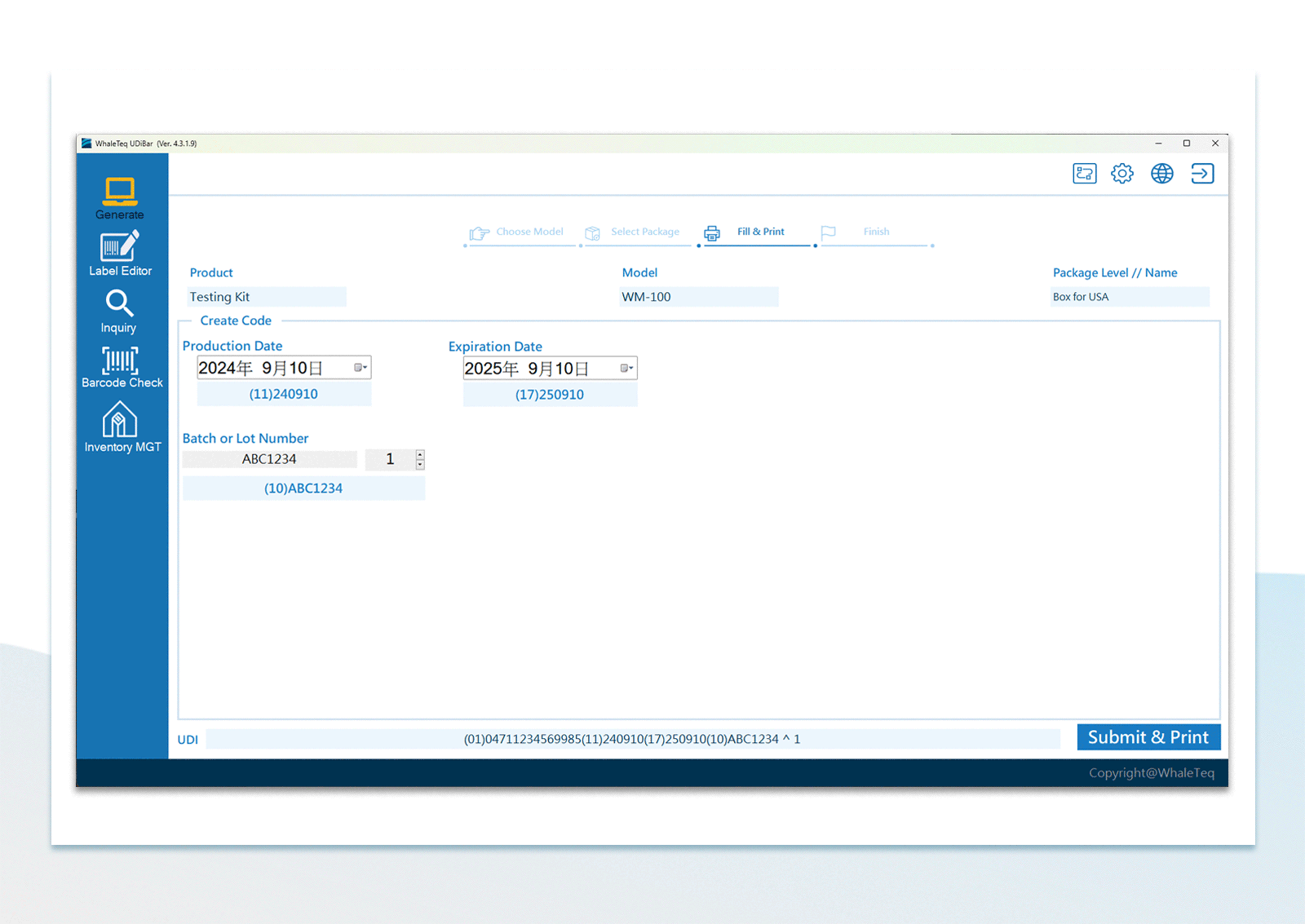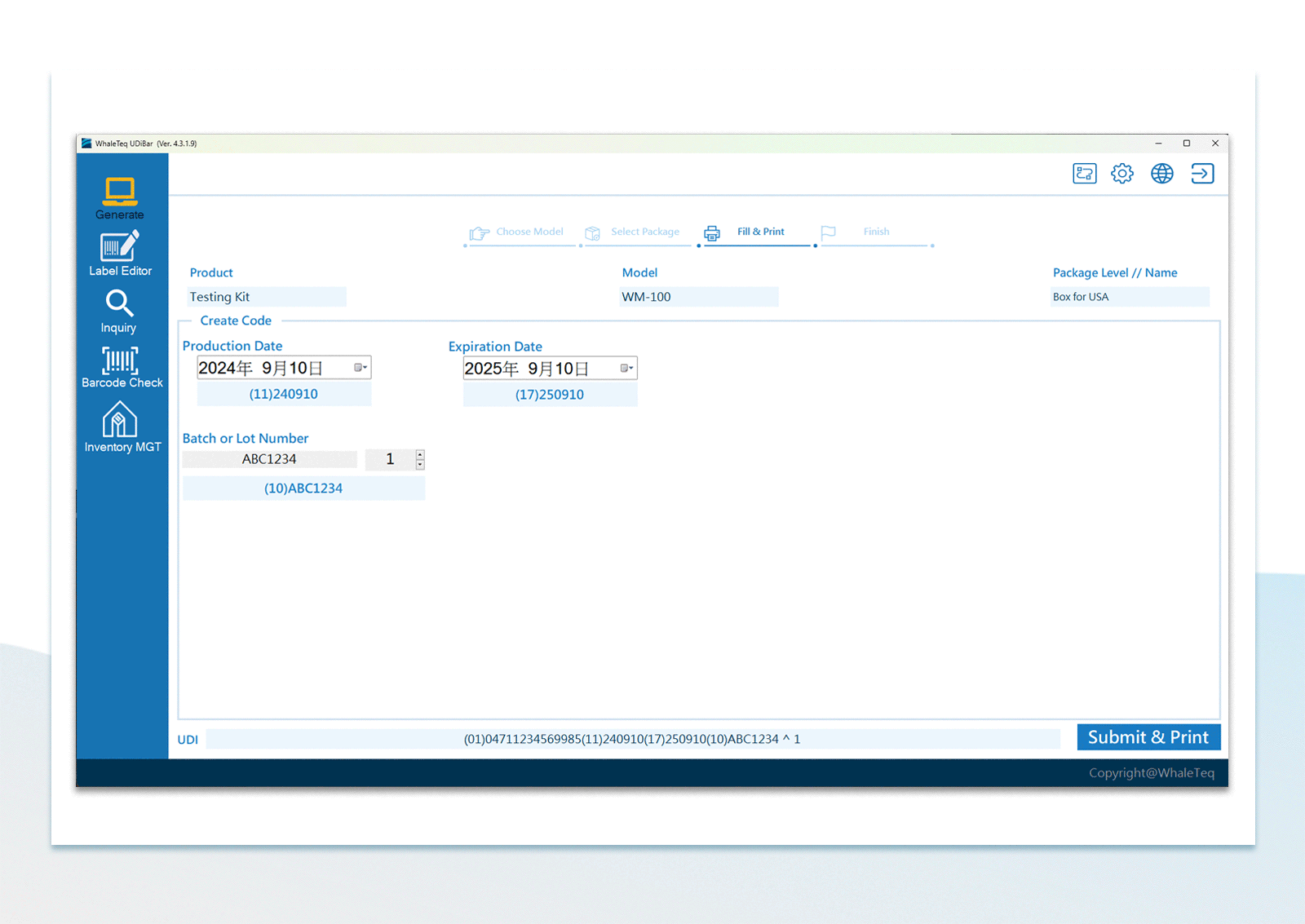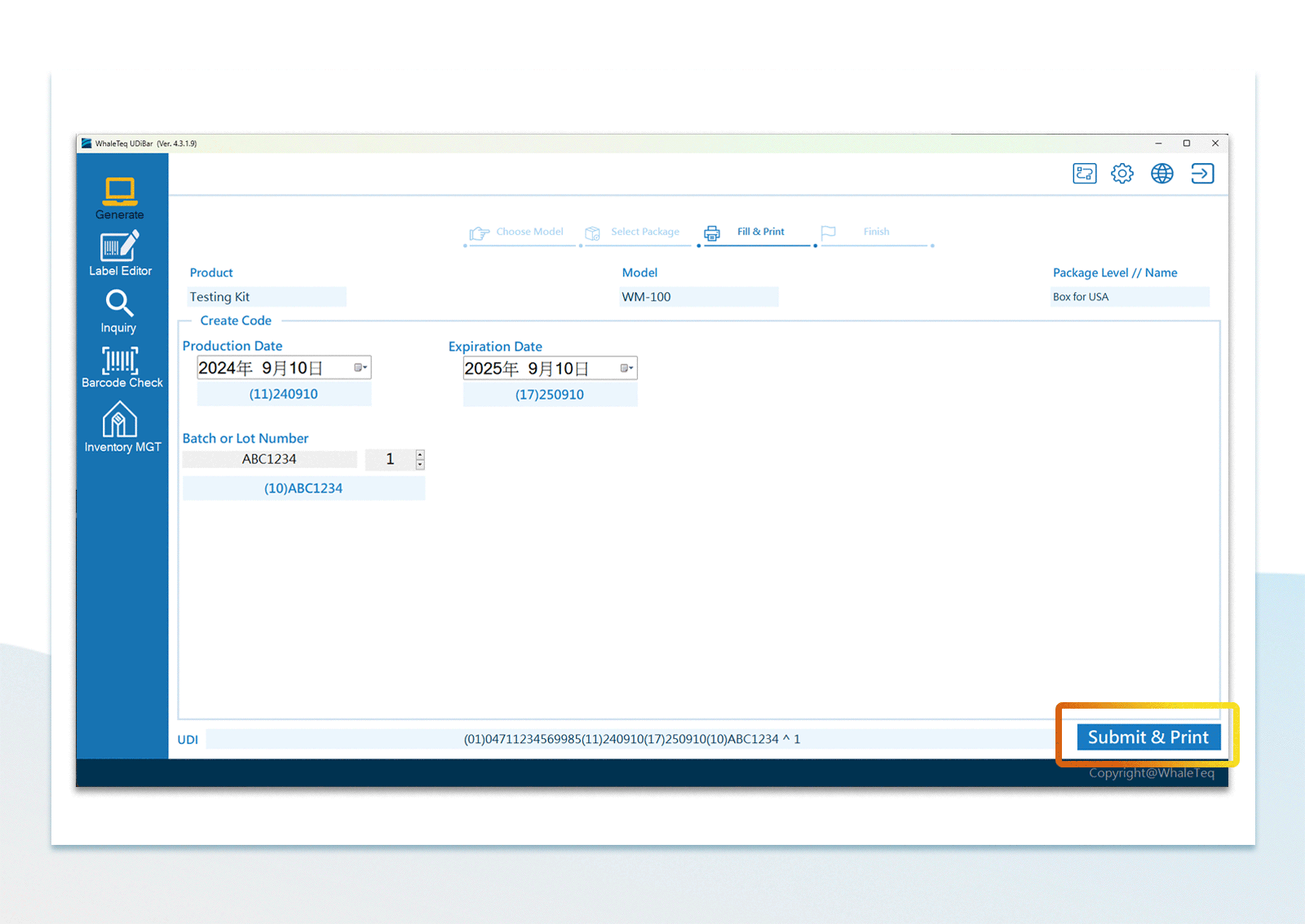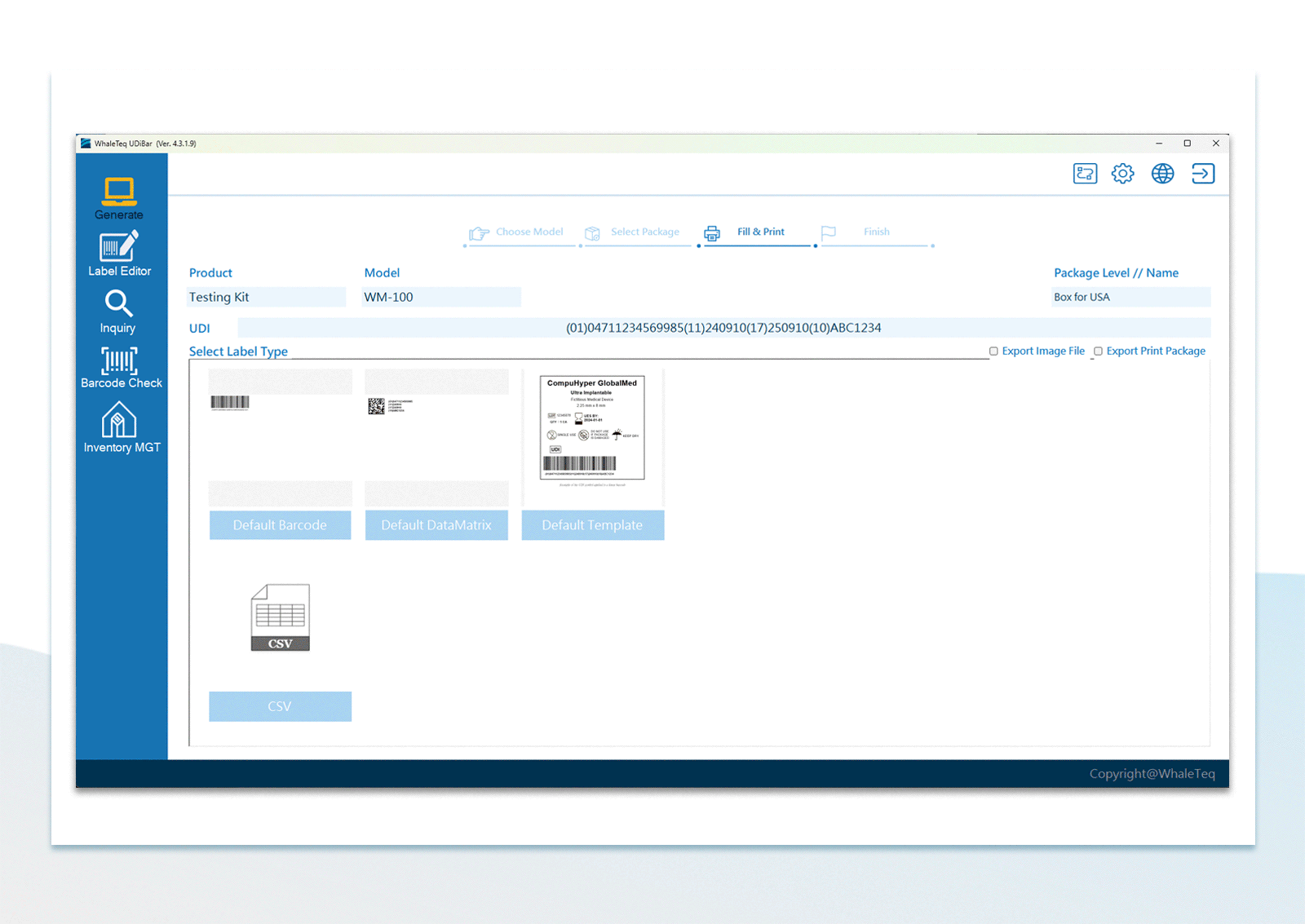
 UDI Encoding and Labeling
UDI Encoding and Labeling UDI Label Printing
UDI Label Printing UDI Database Management
UDI Database Management Flexible Configuration of Database Backup
Flexible Configuration of Database Backup UDI Barcode Check
UDI Barcode Check Inventory Management
Inventory Management