Origins
CMRR is a key indicator of a medical device's ability to withstand electrical noise. Common mode interference is interference signals coming from external power sources, circuits, or other equipment, and medical devices are often used in high-noise environments, where electromagnetic interference, in particular, can impact the accuracy and reliability of devices.
Therefore, medical standards require CMRR testing to ensure that ECG devices continue to display accurate readouts even in noisy environments. ECG device manufacturers also aim to maximize products' CMRR value to produce clearer, more readable ECG signals.
However, it's challenging for manufacturers to create a dedicated CMRR tester that adheres to the specific circuit and hardware design requirements outlined in medical standards. The circuit must replicate various conditions, such as simulating the capacitance effect (100pF) between the patient bed and the floor, providing a voltage of 20Vrms, and including a parallel circuit of 51kΩ/47nF to simulate the impedance between electrodes and skin. These complex requirements increase design difficulty, and maintenance and calibration for the tester further add to the challenges.
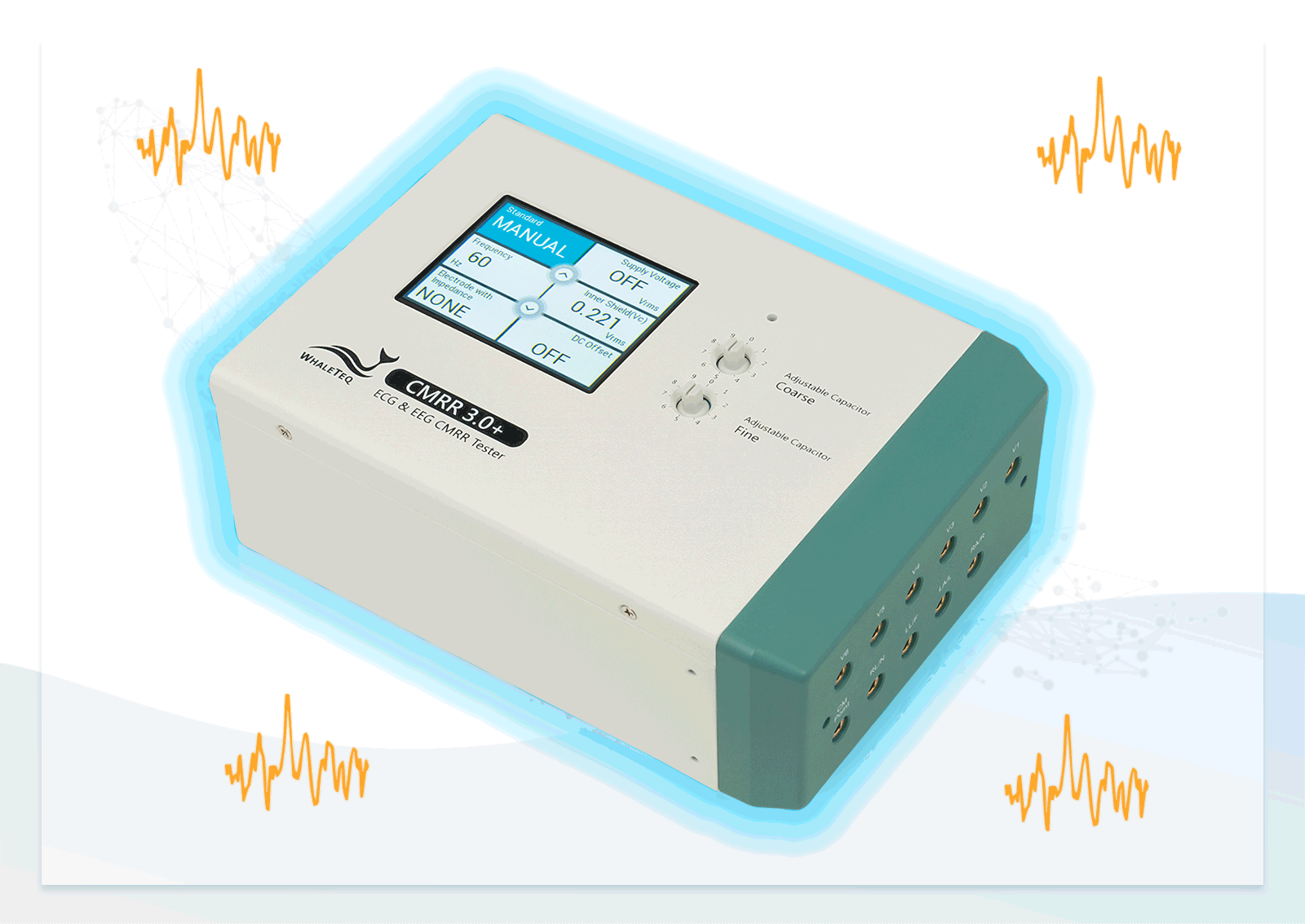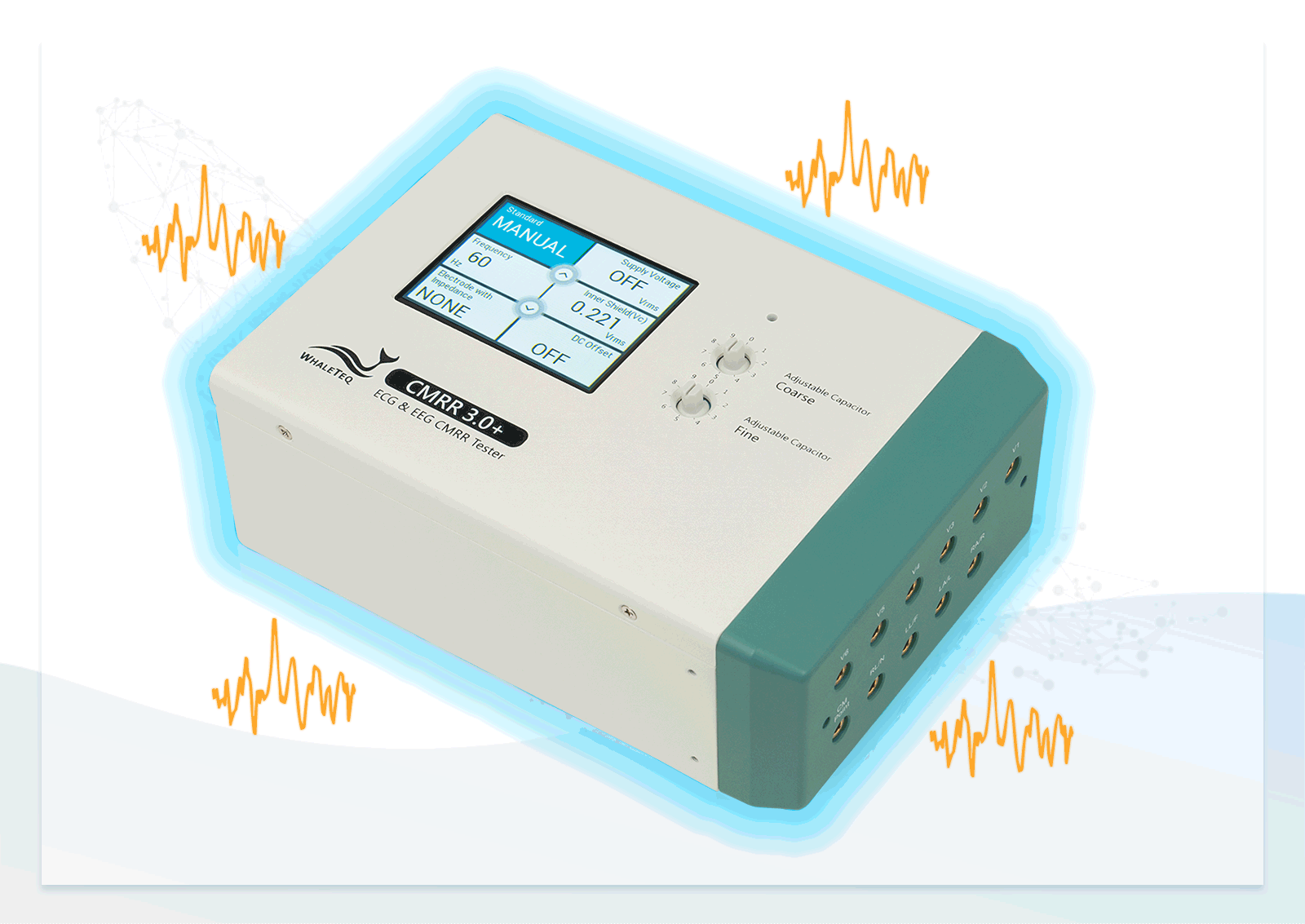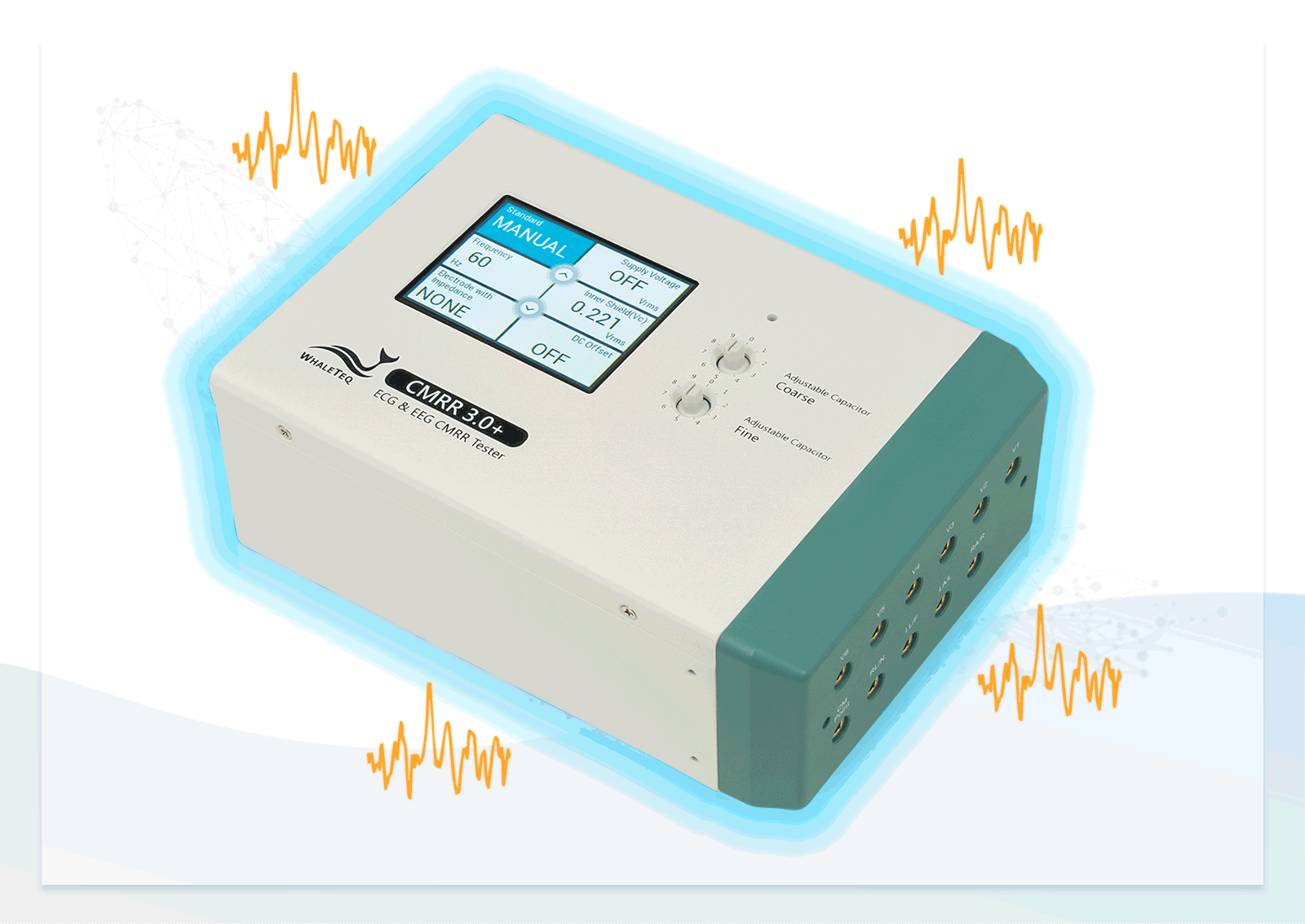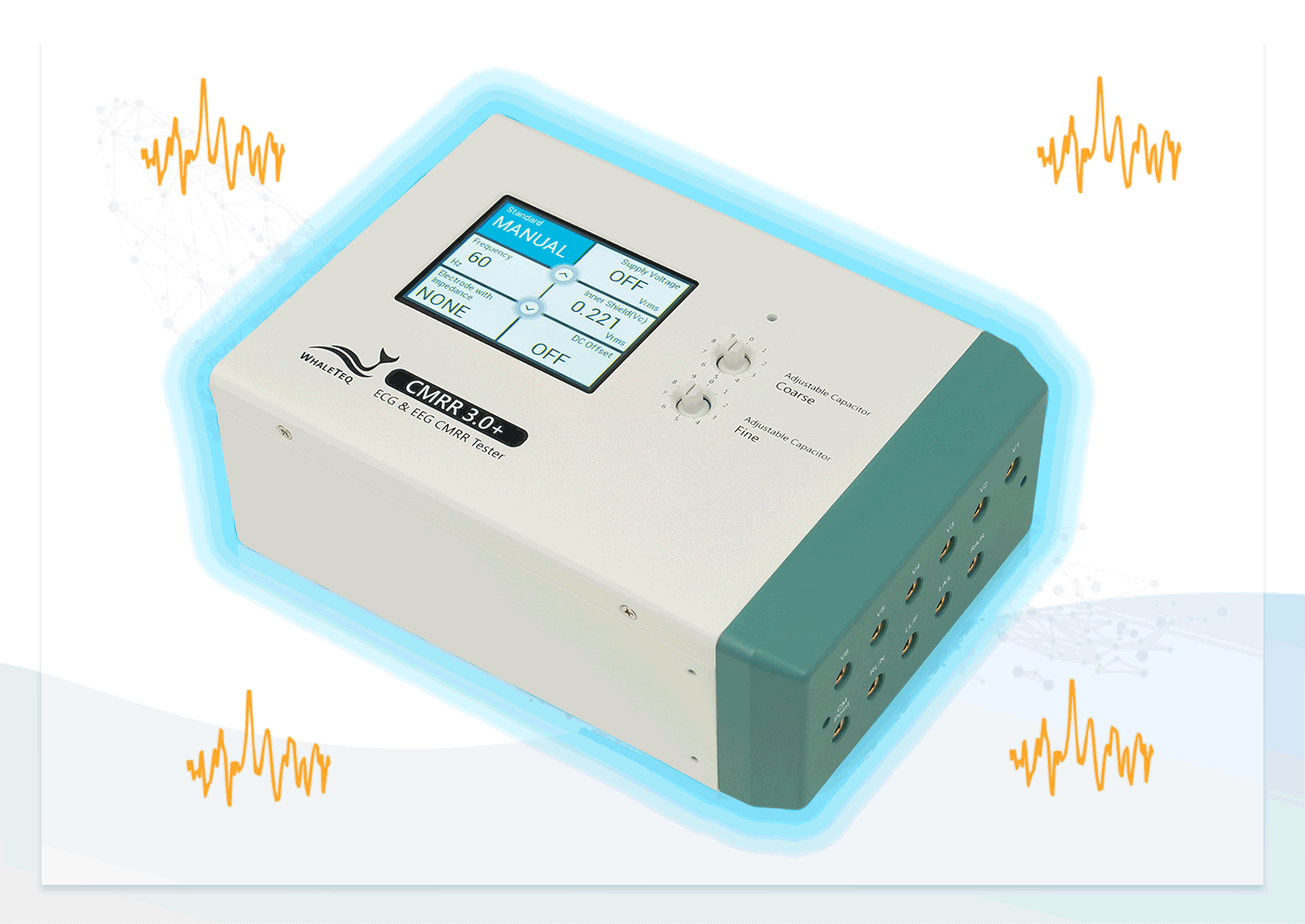
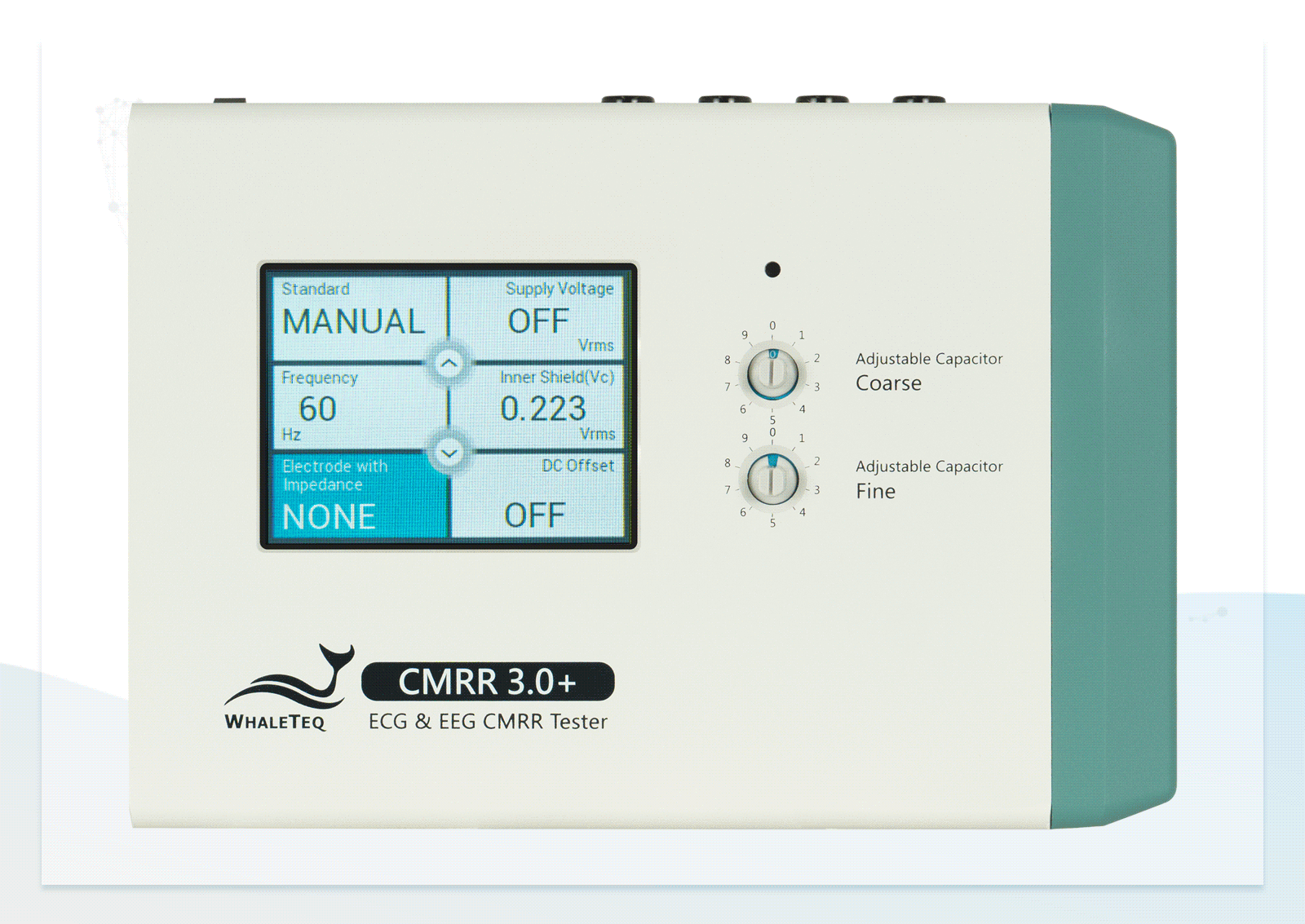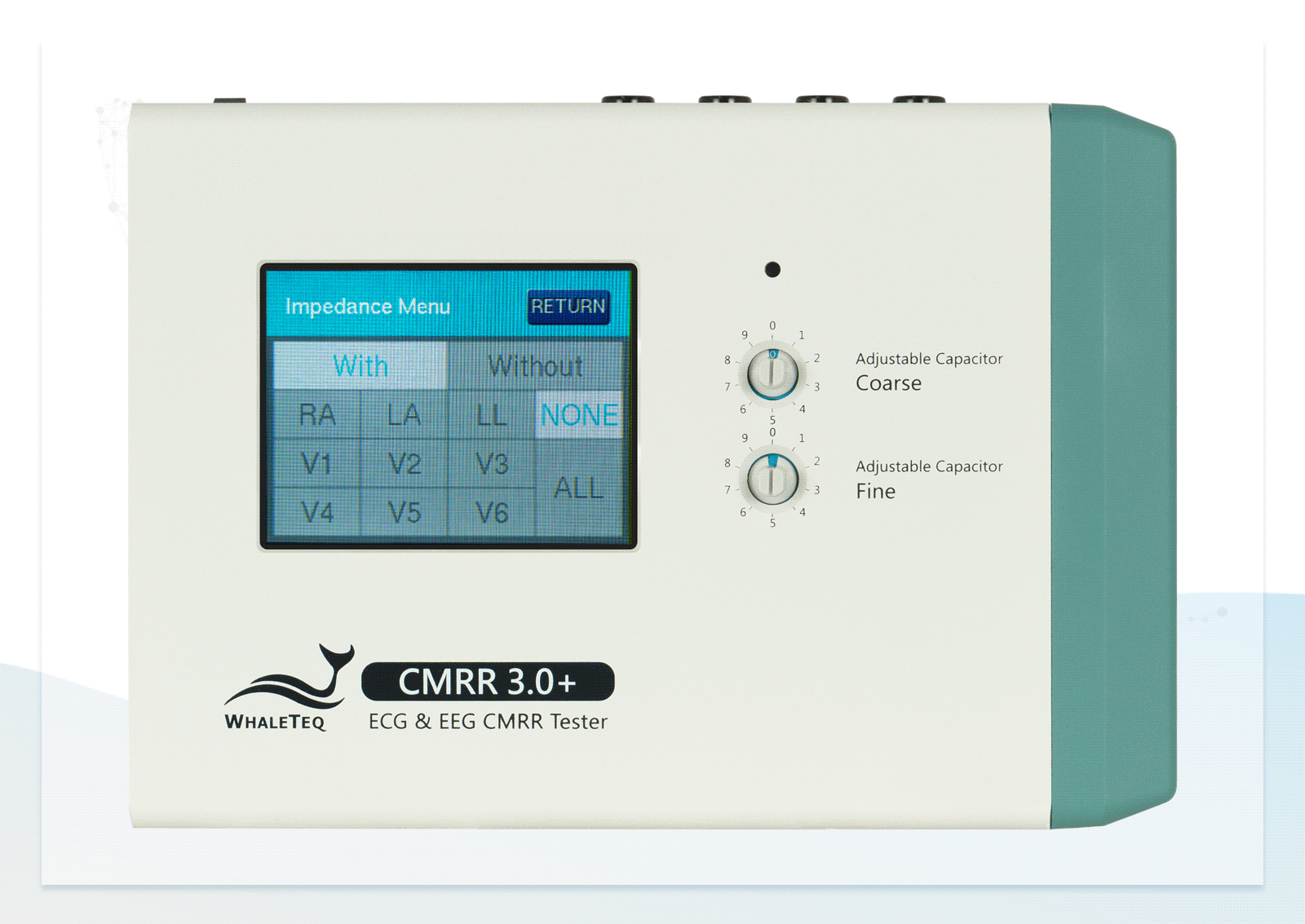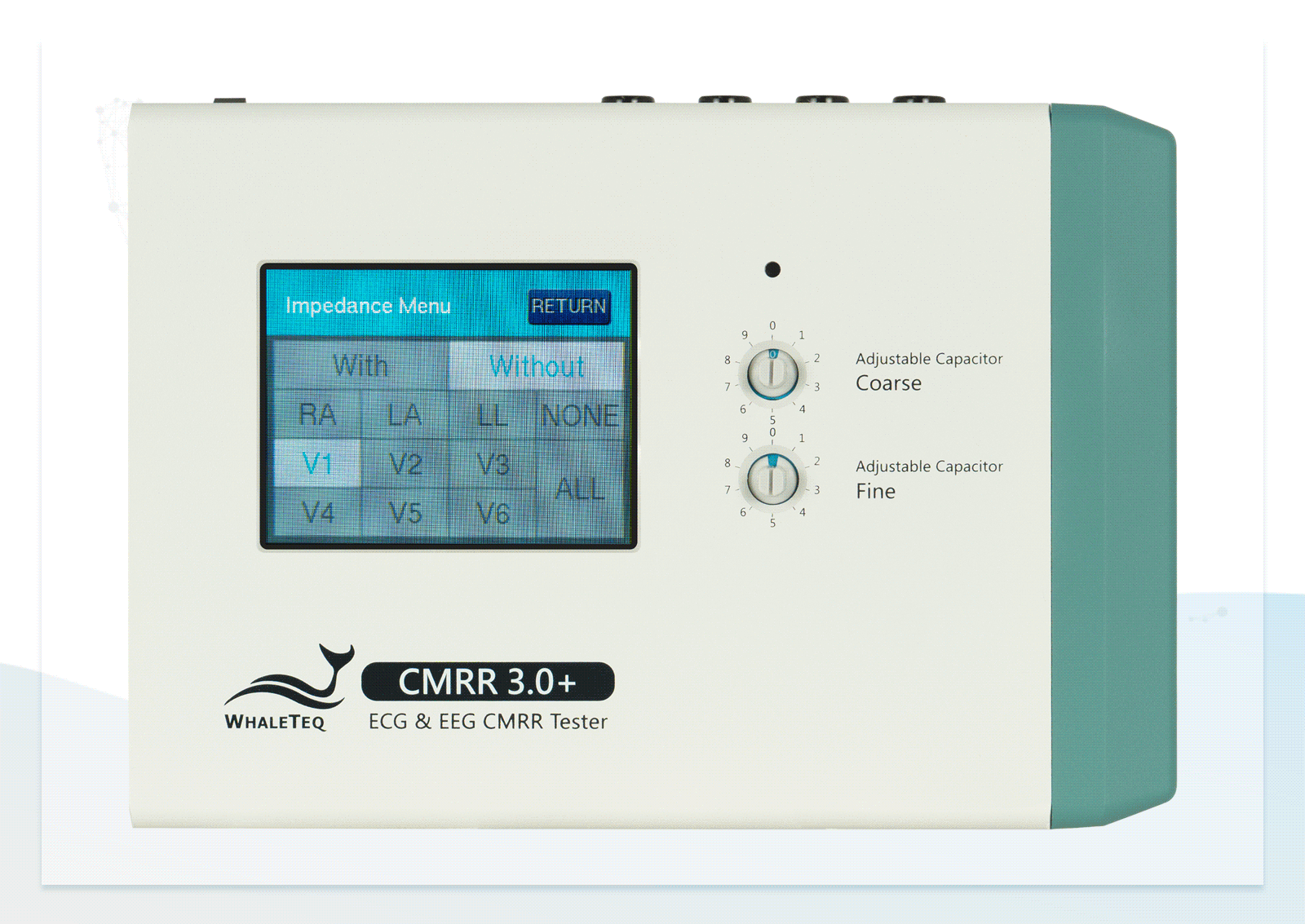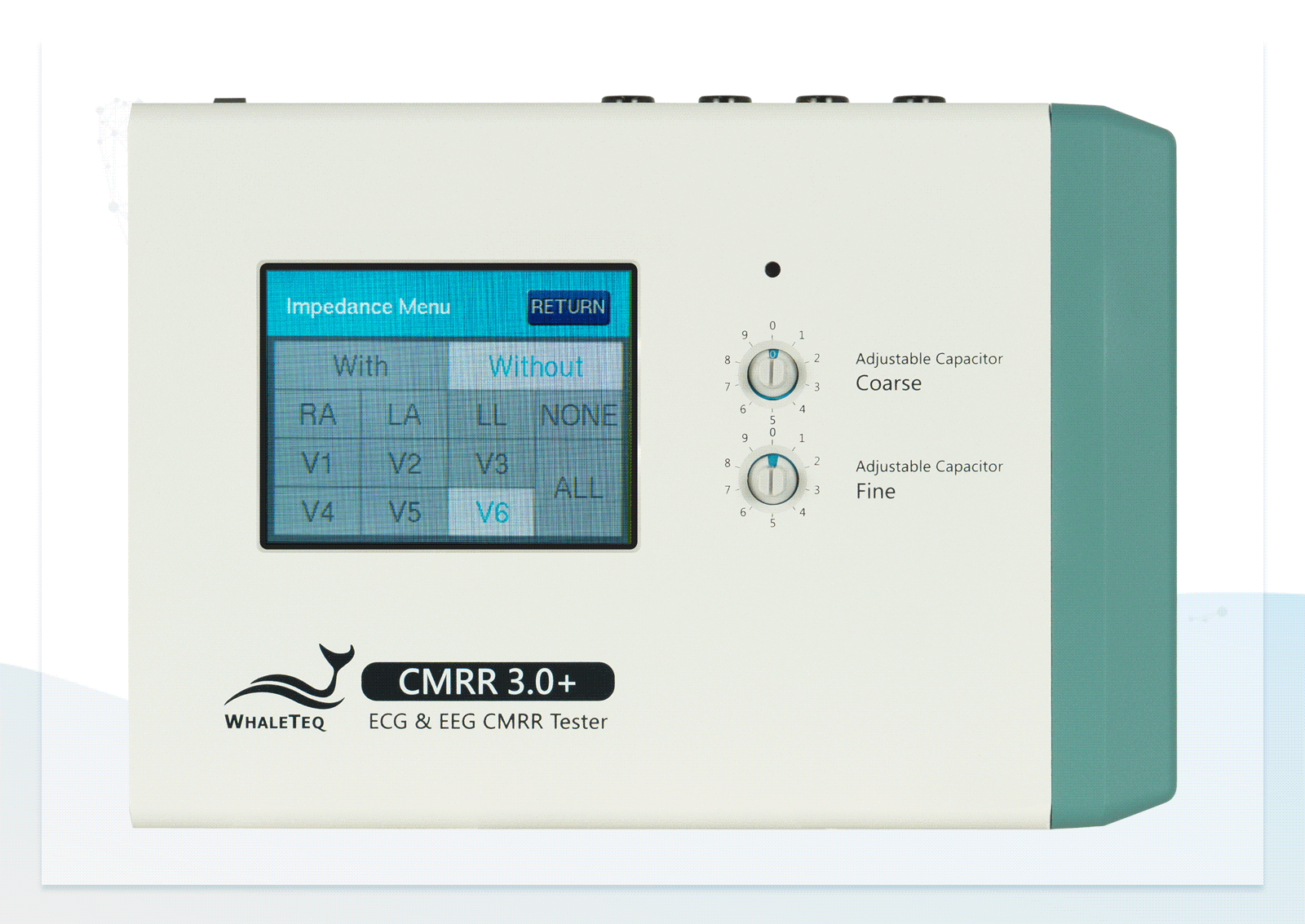
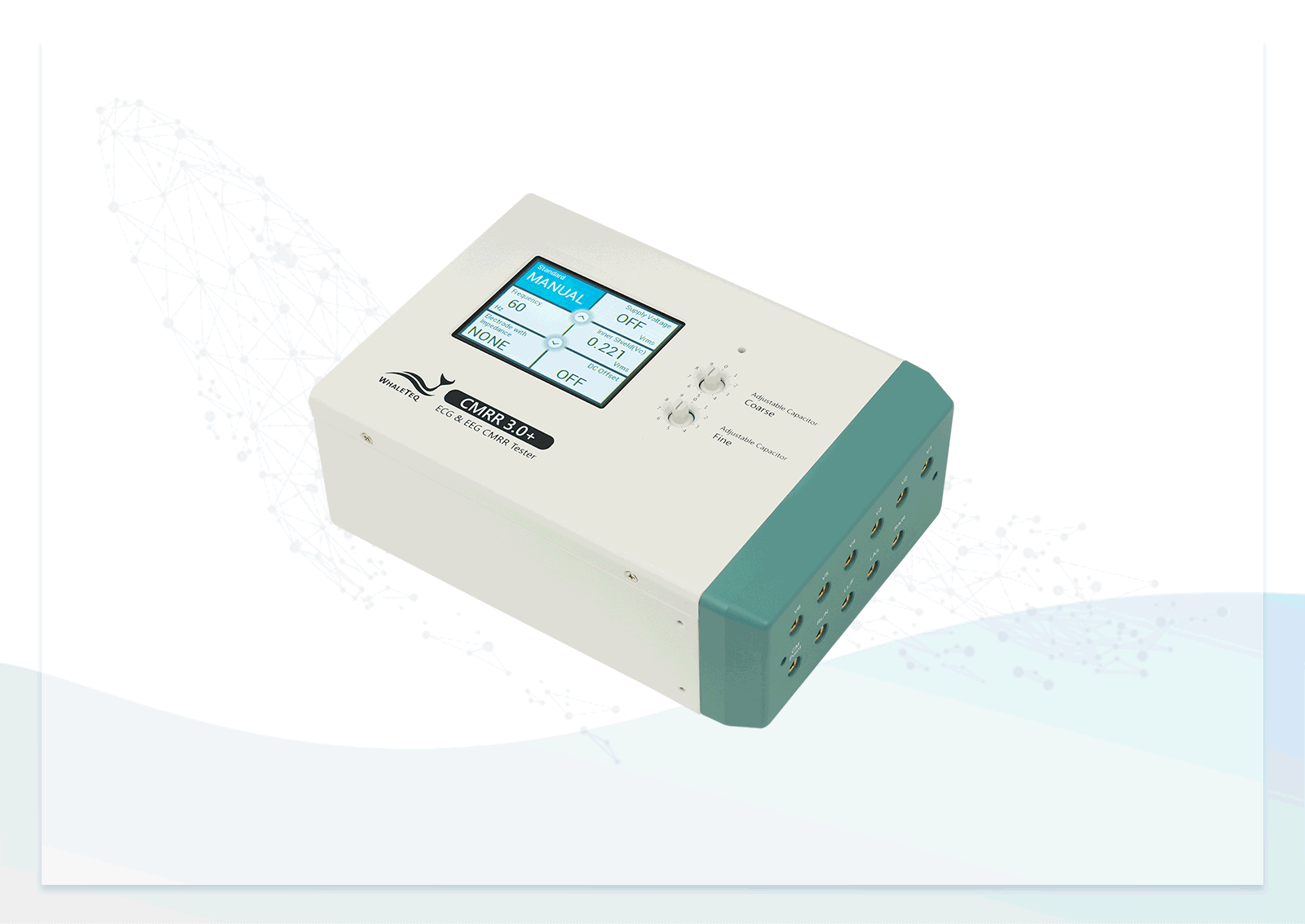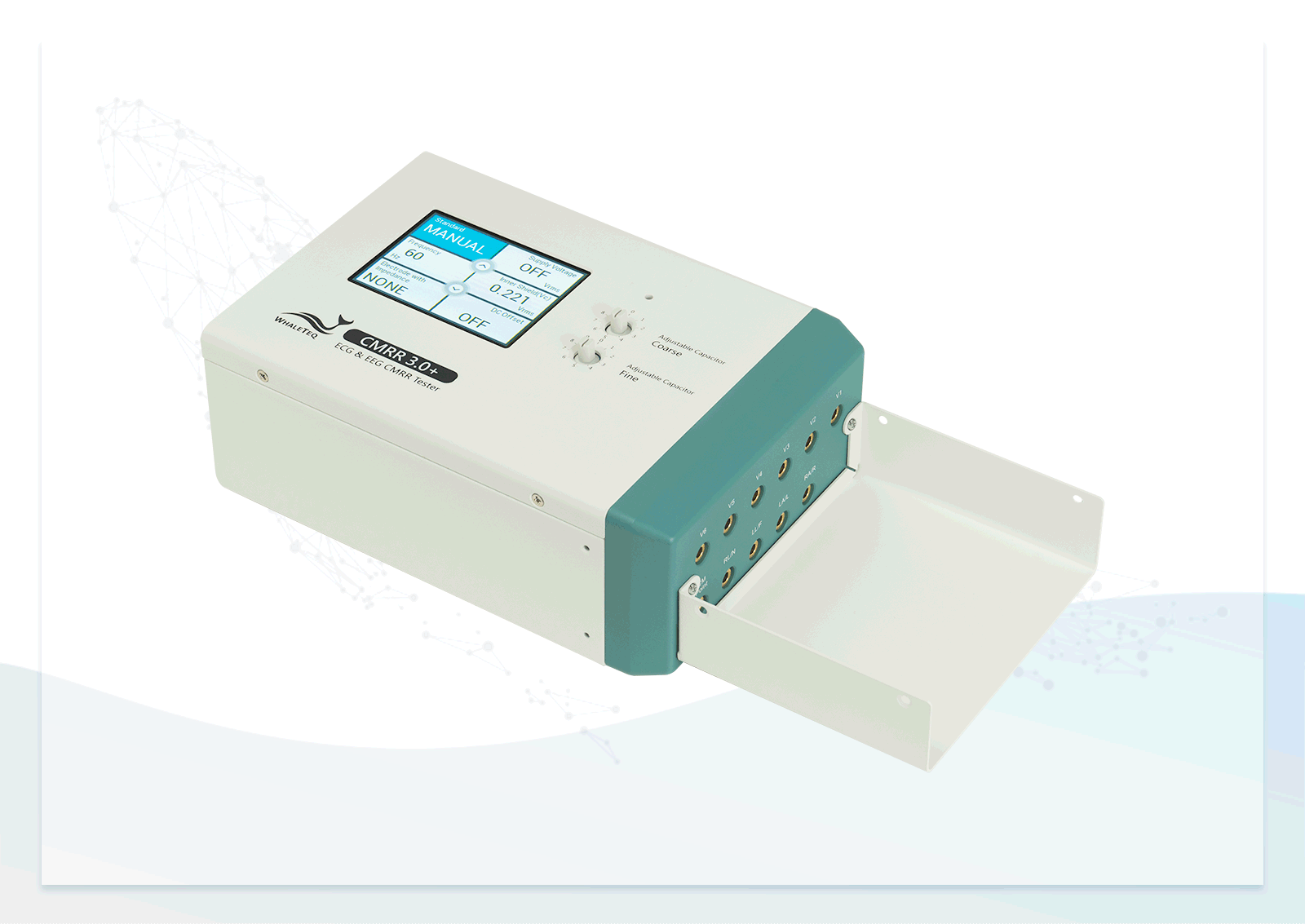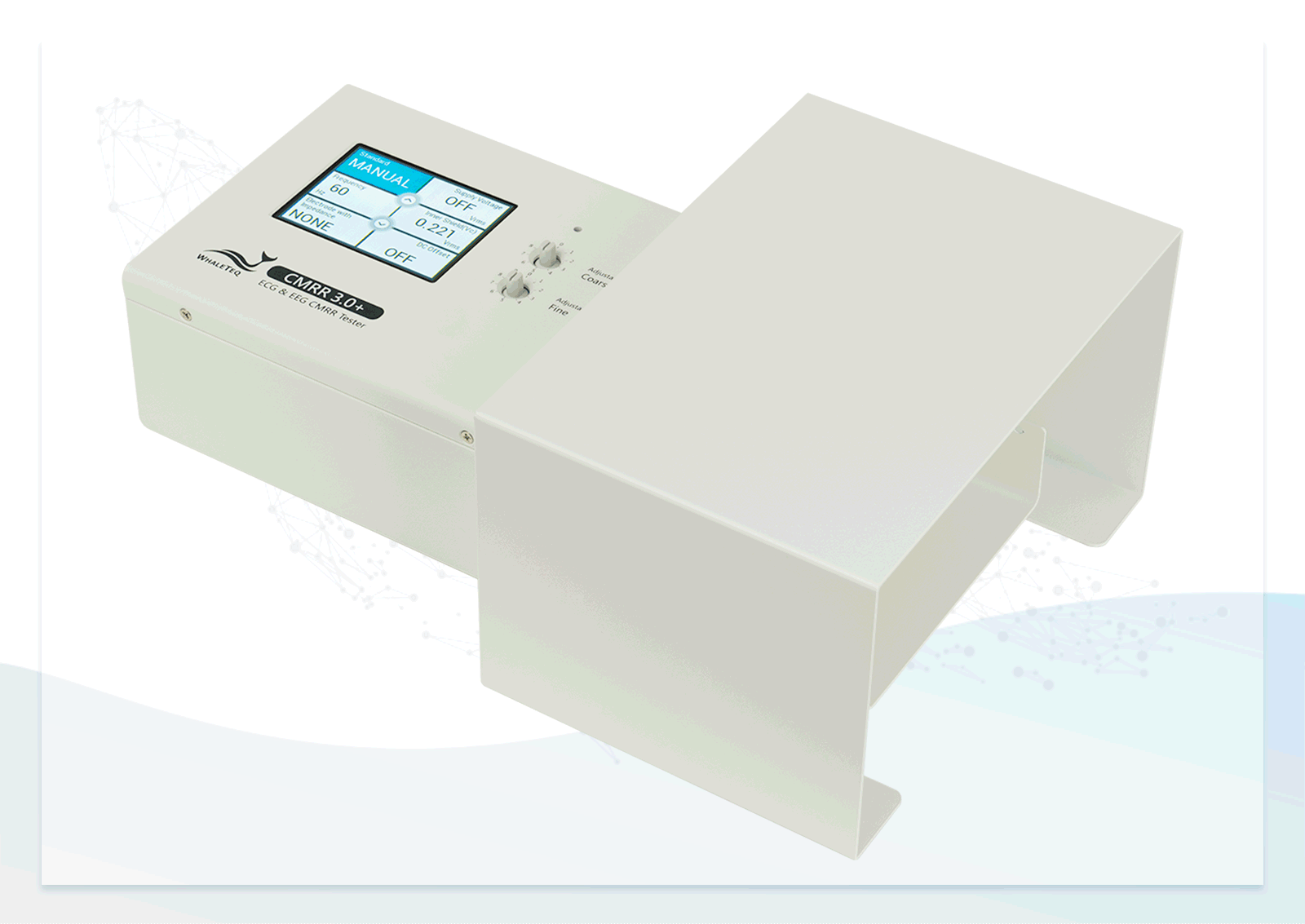
After studying standards and developing the first version, we introduced the CMRR 3.0+. It incorporates a double-layer shielding design for internal circuits to meet regulatory requirements and effectively control noise levels for consistent testing. With a built-in sine wave signal generator and voltage measurement circuit, it eliminates the need for external signal generators, which could introduce extra noise, and ensures sufficient voltage supply for easy test setup.
In addition to providing the standard voltage, the CMRR 3.0+ offers a higher output voltage of up to 70.71Vrms, extending the CMRR test range to 120dB—beyond the 89dB required by standards—helping engineers enhance product specifications.
The CMRR 3.0+ features robust noise isolation, making it suitable for both R&D and production line testing, and when paired with our free software development kit (SDK), it allows efficient automated testing in production.
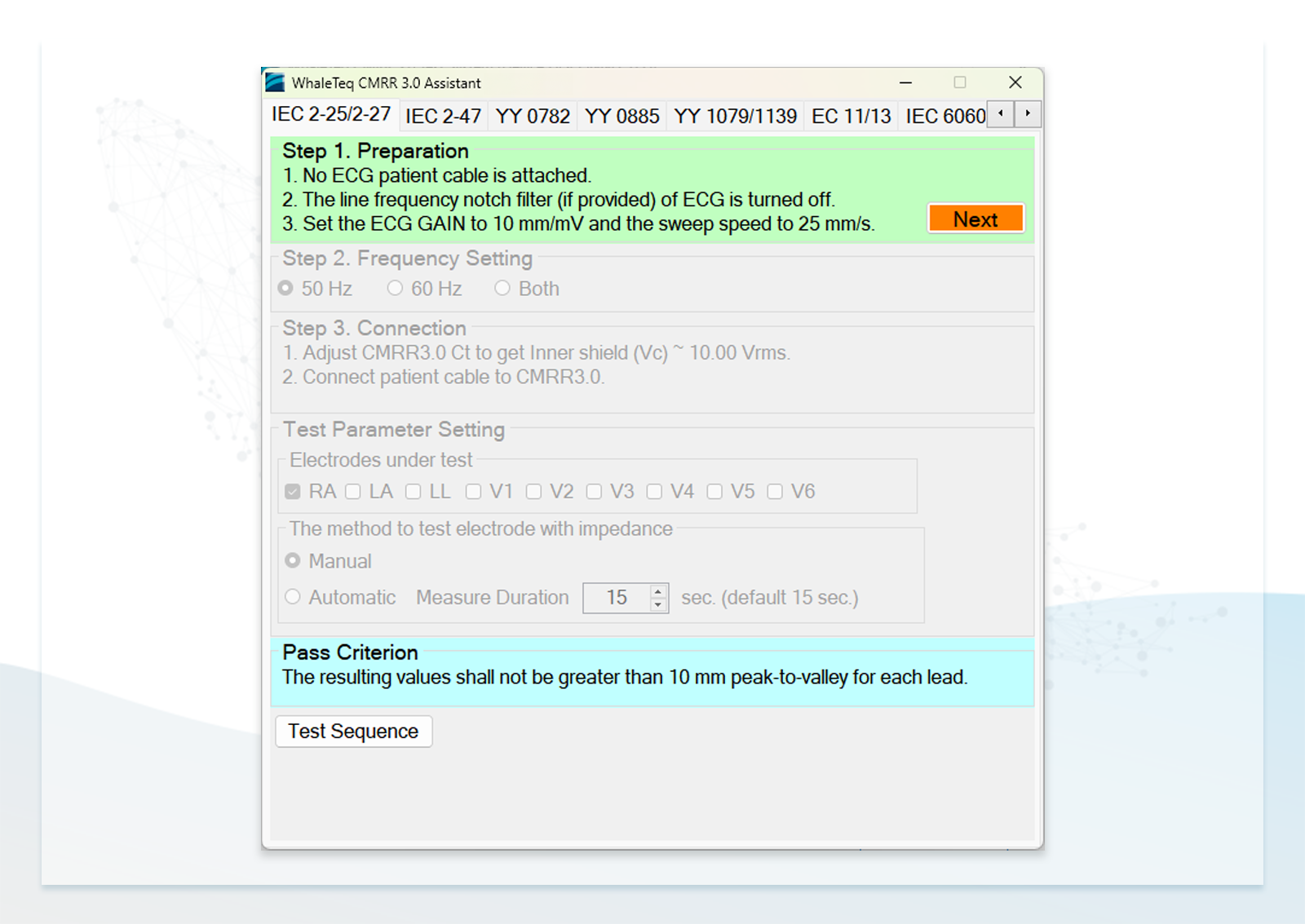
To further assist manufacturers in compliance testing, we offer exclusive Standard Assistant Software that simplifies the setups of standard test items. Users can simply follow the instructions and select the parameters to easily complete compliance testing.
CMRR forms one of the foundational aspects of medical device excellence. We hope the CMRR 3.0+ can help manufacturers build superior ECG devices by providing reliable and high-quality performance.
Functions
Highlights
All-in-One Circuit Design to Overcome Testing and Technical Challenges
- Built-in calibrated sine wave signal generator solves the common issue of insufficient voltage supply from general signal generators
- Built-in calibrated voltage measurement circuit allows real-time monitoring of the common mode voltage (Vc) to ensure it is half the value of the signal generator's voltage (Vs) when adjusting the variable capacitor
- Built-in MCU and electronic relays enable manual or automatic switching of RC circuits and voltage options
High CMRR Testing to Enhance Product Competitiveness
- Double-layer shielding structure for minimizing both internal and external noise interference
- Capable of achieving up to 111dB for CMRR in unbalanced testing
- Shielding covers can be added to enable testing for higher CMRR values
Provided SDK for Flexibly Developing Programs as Needed
- Users can develop automated testing software based on their needs to control the testing process, reducing the effort of repetitive setups
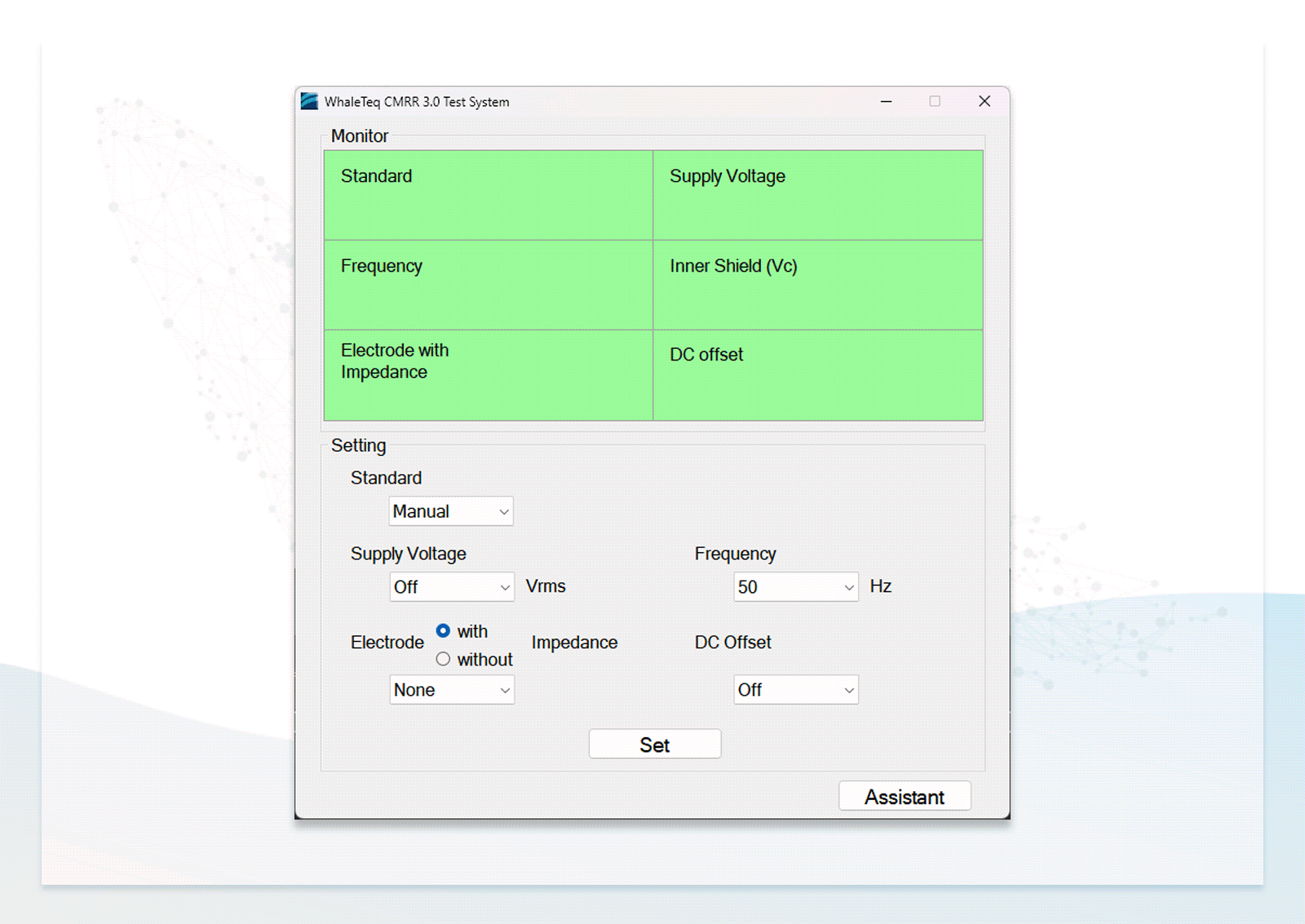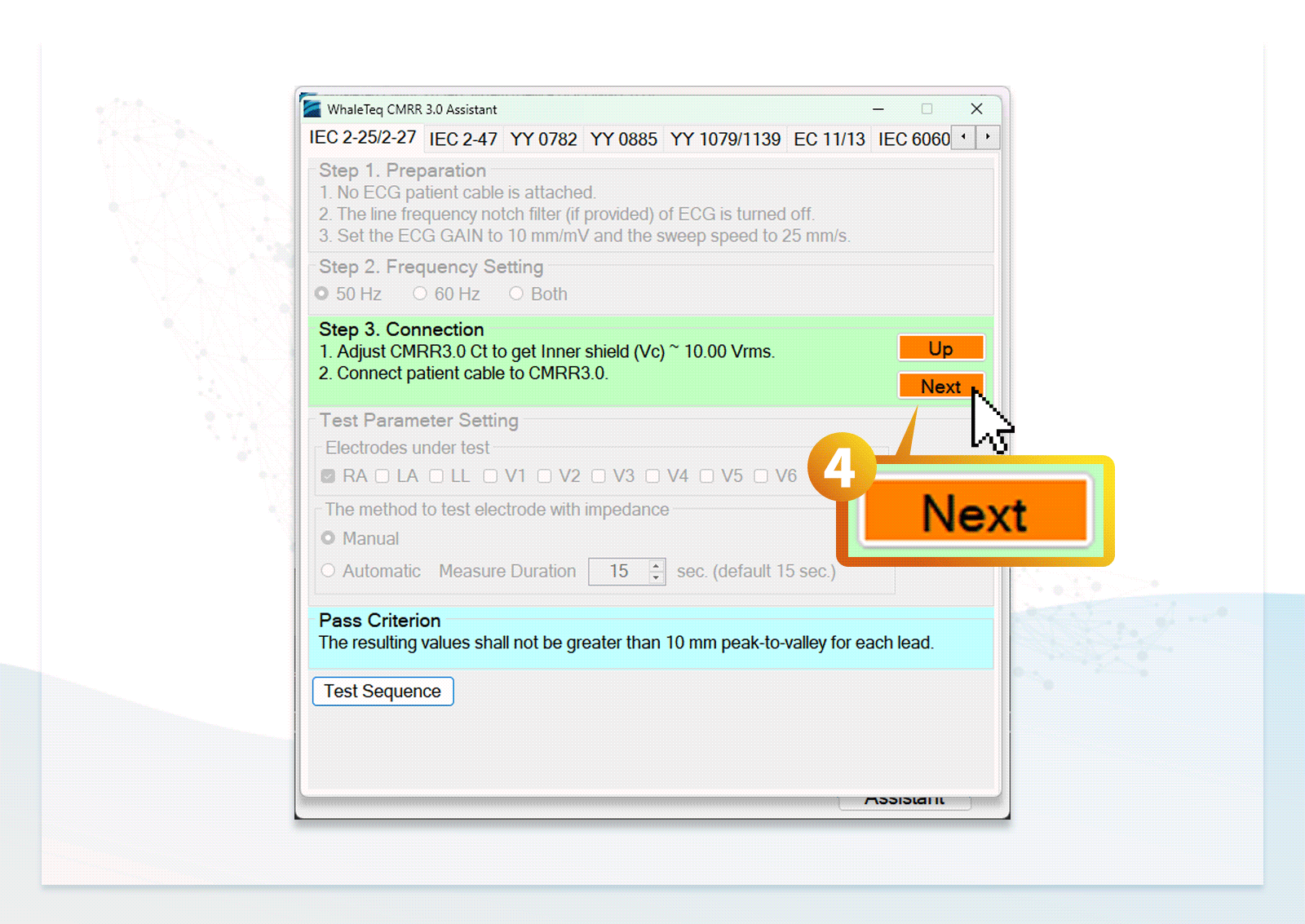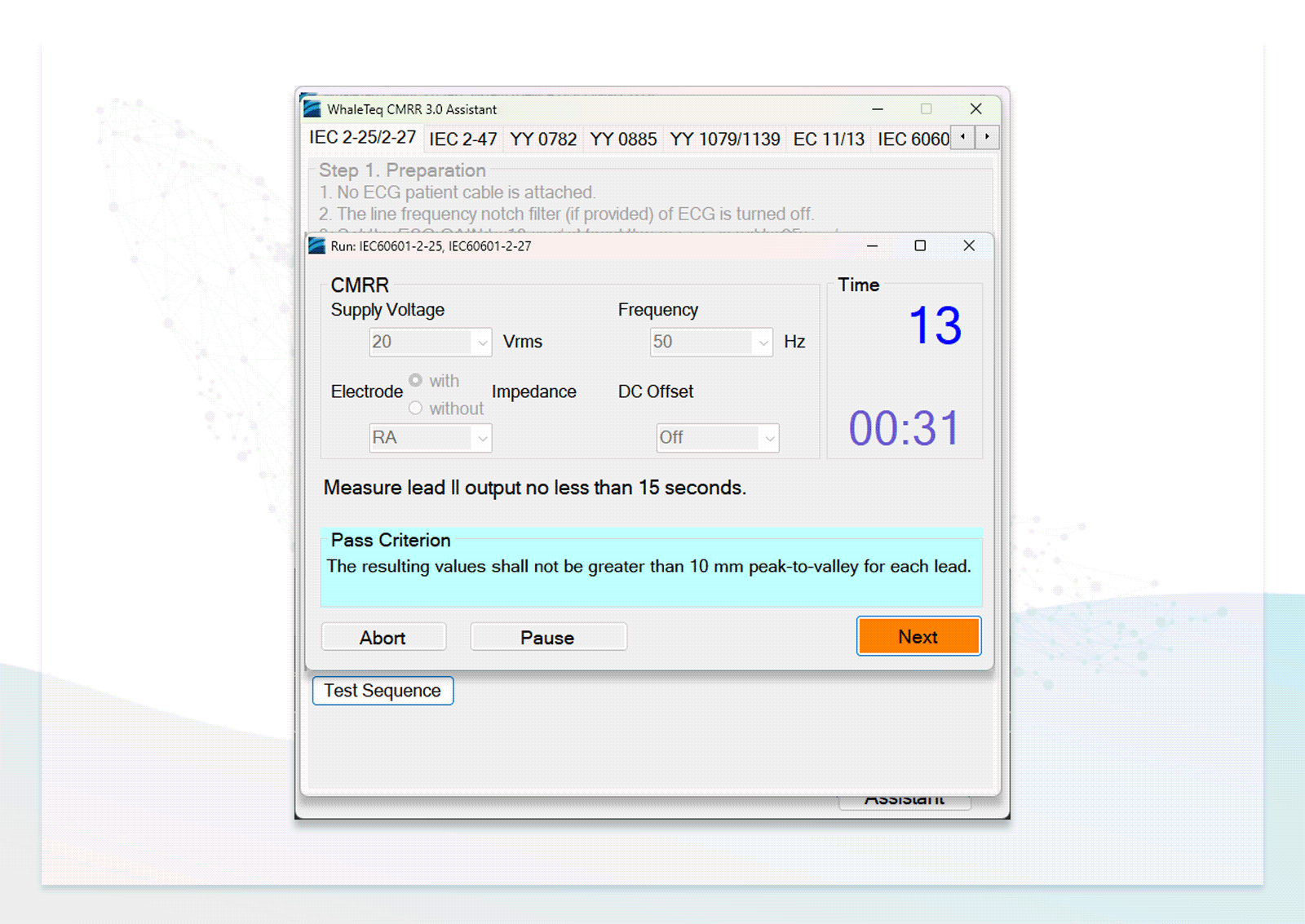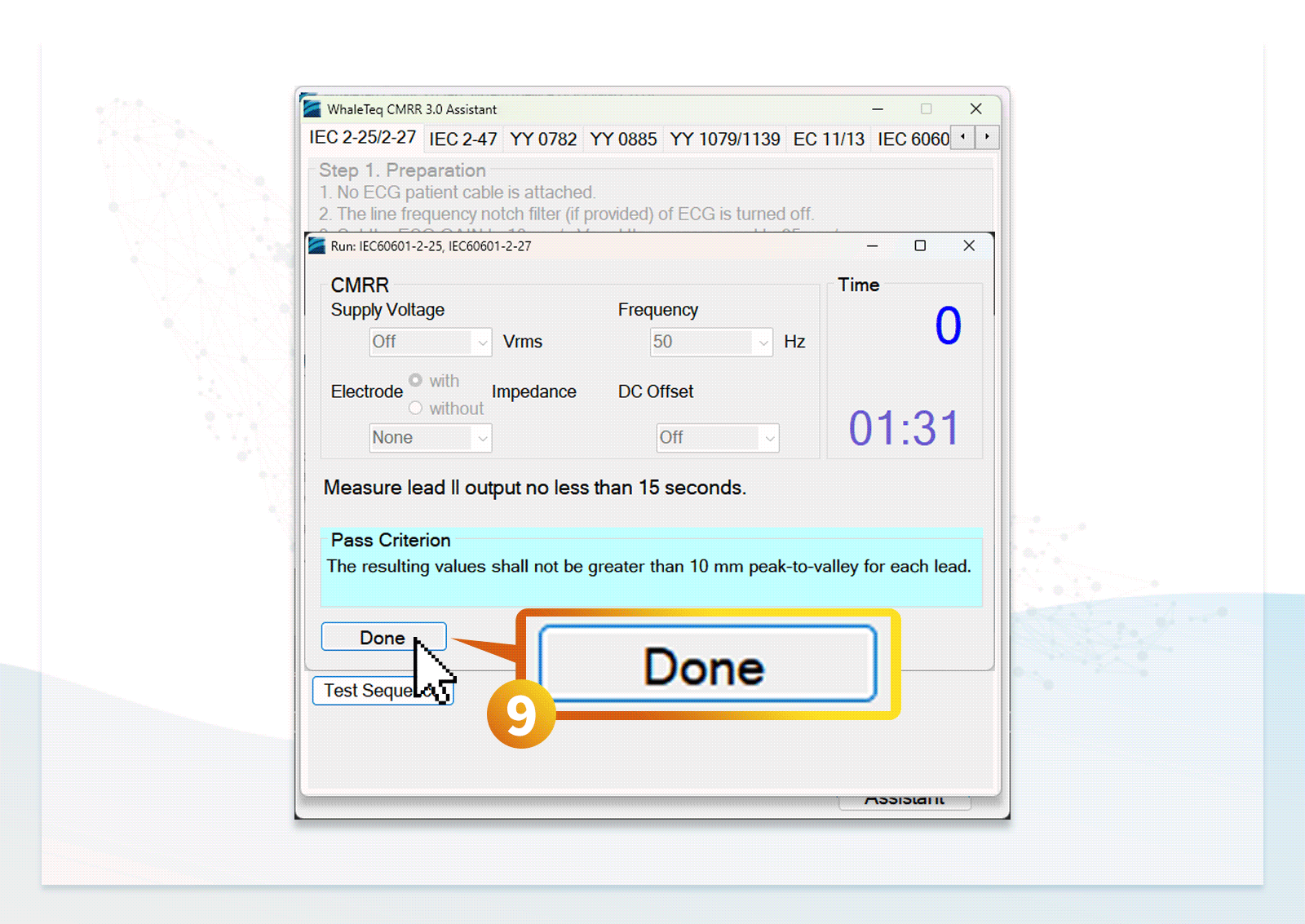
Standard Assistant Software for Effortlessly Conducting Compliance Tests
- Supports ECG-related international standards IEC 60601-2-25, IEC 60601-2-27, and IEC 60601-2-47, EEG-related international standard IEC 60601-2-26, as well as Chinese regulations GB 9706.225, GB 9706.227, YY 9706.247, GB 9706.226, YY1079, YY1139, YY0782, and YY0885
- Users can follow the step-by-step instructions in the standard assistant software to configure the DUT, select parameters, and conduct compliance testing
Note: The Standard Assistant Software needs a separate purchase.
Comprehensive Compliance Testing Solution
-
Saves time studying the medical standard and training by completing performance and database testing required by IEC standards with WhaleTeq ECG testing products
👉IEC 60601-2-25 Testing Package
👉IEC 60601-2-27 Testing Package
👉IEC 60601-2-47 Testing Package
| Items | Options | Specifications |
|---|---|---|
| Supply voltage | 0.5 / 2.828 / 20 / 2 / (70.71) (Vrms) | ±1%* |
| CM point voltage | 1.414 / 10 / 1 / (35.355) (Vrms) | ±1% (0.25Vrms ±2%) |
| Frequency | 50 / 60 / 100 / 120 (Hz) | ±1% |
| Electrode with Impedance | Change electrode via the touch screen of CMRR 3.0+ | None / RA / LA / LL / V1 / V2 / V3 / V4 / V5 / V6 / ALL |
| Electrode without Impedance | Change electrode via the touch screen of CMRR 3.0+ | RA / LA / LL / V1 / V2 / V3 / V4 / V5 / V6 |
| Imbalance impedance, R | 51kΩ | 51kΩ ±1% |
| Imbalance impedance, C | 47nF | 47nF ± 5% |
| DC offset | Powered by a replaceable preinstalled battery, can be added on RA / LA / LL / V1~V6 | 300mV ± 1% Up to 40hrs with intermittent use |
| 100pF capacitor | Use 11:1 (110MΩ:10MΩ) voltage divider to measure indirectly | 100pF ± 5% |
| Environment | Intended for normal laboratory environment. The selection of critical components is known to be stable in the range shown. The 110MΩ divider may be affected by high humidity in excess of 85%. | 15 - 30°C 10 - 75% RH |
*Require to measure with the multimeter of higher accuracy.

【Case Study】Automated Sequential Testing for Single-lead ECGs

【Case Study】Automated Test Solution for GE Patient Monitor
CMRR 3.0+ Test System
| Part No. | Description | Quantity |
|---|---|---|
| 100-CM00003 | Model No.: CMRR 3.0+ Advanced common mode rejection ratio tester with 11 compound terminals Package contents: • CMRR 3.0+ x 1 • Top shielding cover x 1 • Bottom shielding cover x 1 • Compound terminal x 11 • USB cable x 1 • Grounding wire x 1 • 12V Power adapter x 1 (excluding power cord) | 1 |
Optional Software Add-on Pack
| Part No. | Description |
|---|---|
| HA0-CM04001 | Auto setup for CMRR tests of IEC 60601-2-25 / IEC 60601-2-27 / IEC 60601-2-47 |
| HA0-CM04002 | Auto setup for CMRR tests of YY0782 / YY0885 / YY1079 / YY1139 and ANSI/AAMI EC11 / EC13 standards |
| HA0-CM04003 | Auto setup for CMRR tests of IEC 60601-2-26 |
| HA0-CM04005 | Auto setup for CMRR tests of GB 9706.225 / GB 9706.227 / YY 9706.247 / GB 9706.226 |
Optional Accessories
| Part No. | Description | Quantity |
|---|---|---|
| 100-CM00005 | Model No.: CM30ESBX CMRR 3.0+ External case (30 x 20 x 13cm) for testing Holter with better shielding performance | 1 |
Optional Calibration Service and Warranty Extension
| Part No. | Description |
|---|---|
| YY0007 | Model No.: C3 Provides (3) years of calibration service coverage. WhaleTeq equipment can be calibrated to original performance on the basis of (1) year interval. |
| YY0008 | Model No.: R3 Extends the limited warranty from (1) year to (3) years. |
 Software
Software Datasheet
Datasheet User Manual
User Manual





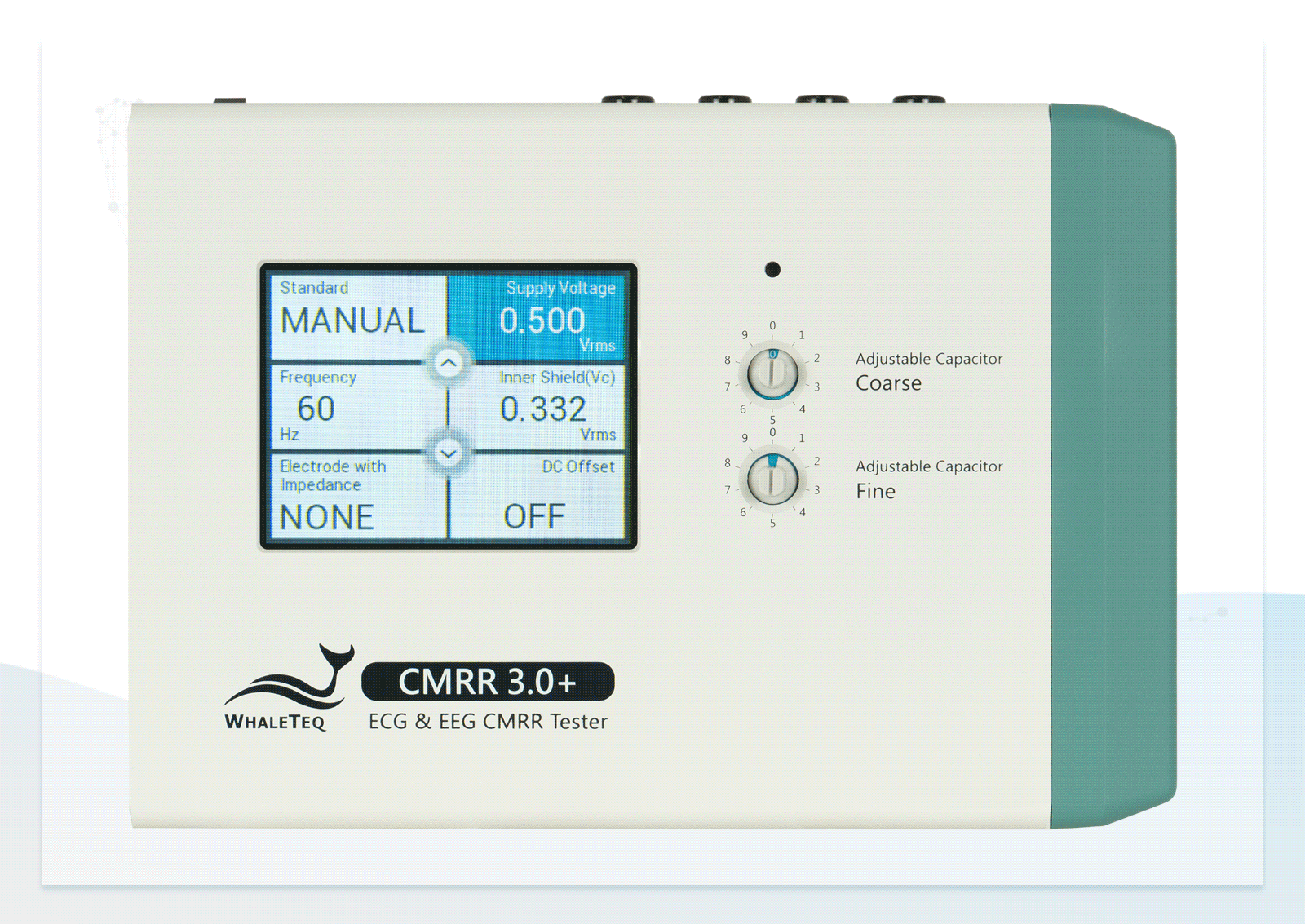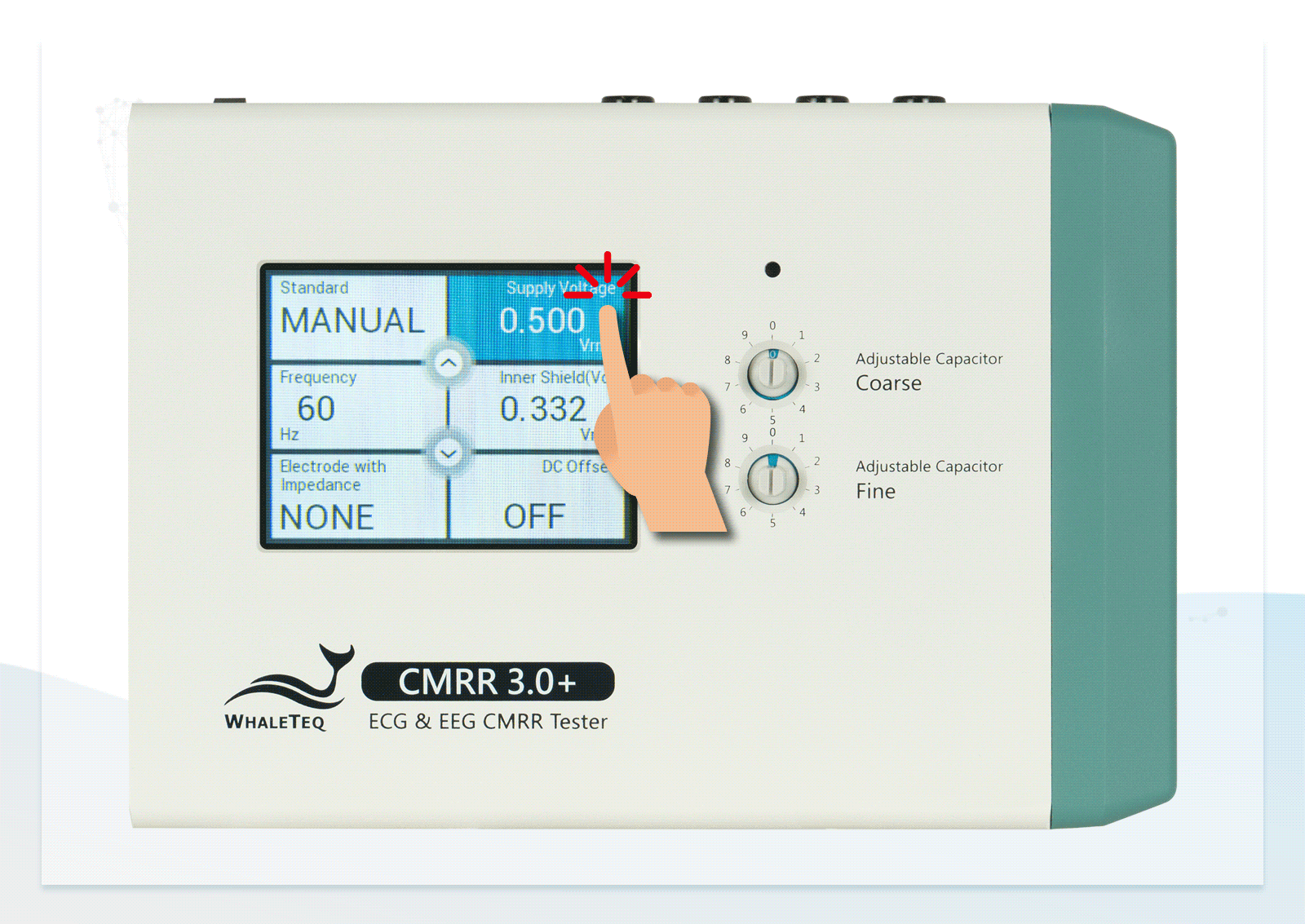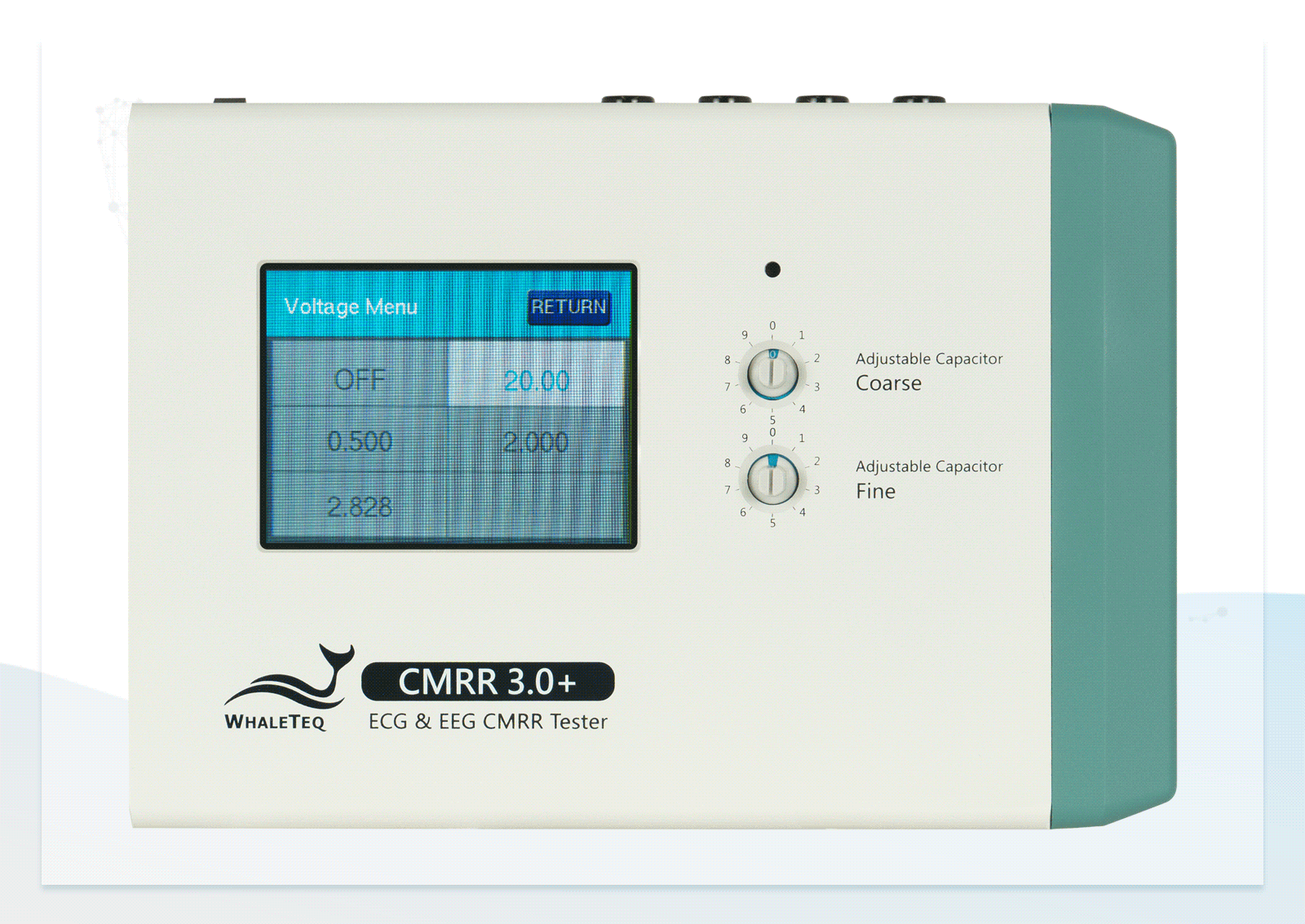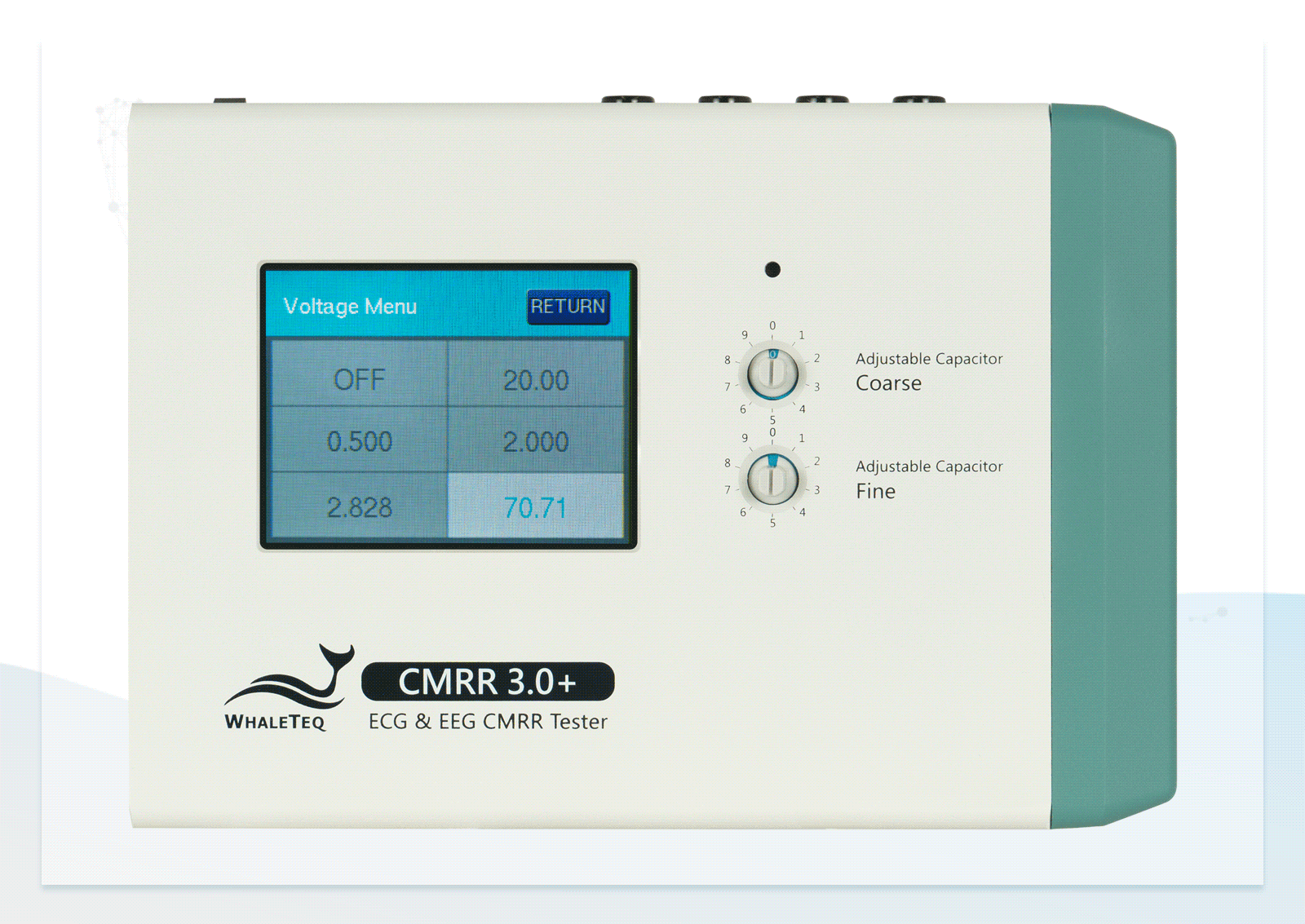
 Built-in Multiple Circuit Design
Built-in Multiple Circuit Design Double-layer Shielding Structure
Double-layer Shielding Structure Wide Voltage Testing Range
Wide Voltage Testing Range Touchscreen for Easily Switching between Electrodes
Touchscreen for Easily Switching between Electrodes Automated Testing
Automated Testing Standard Assistant Software
Standard Assistant Software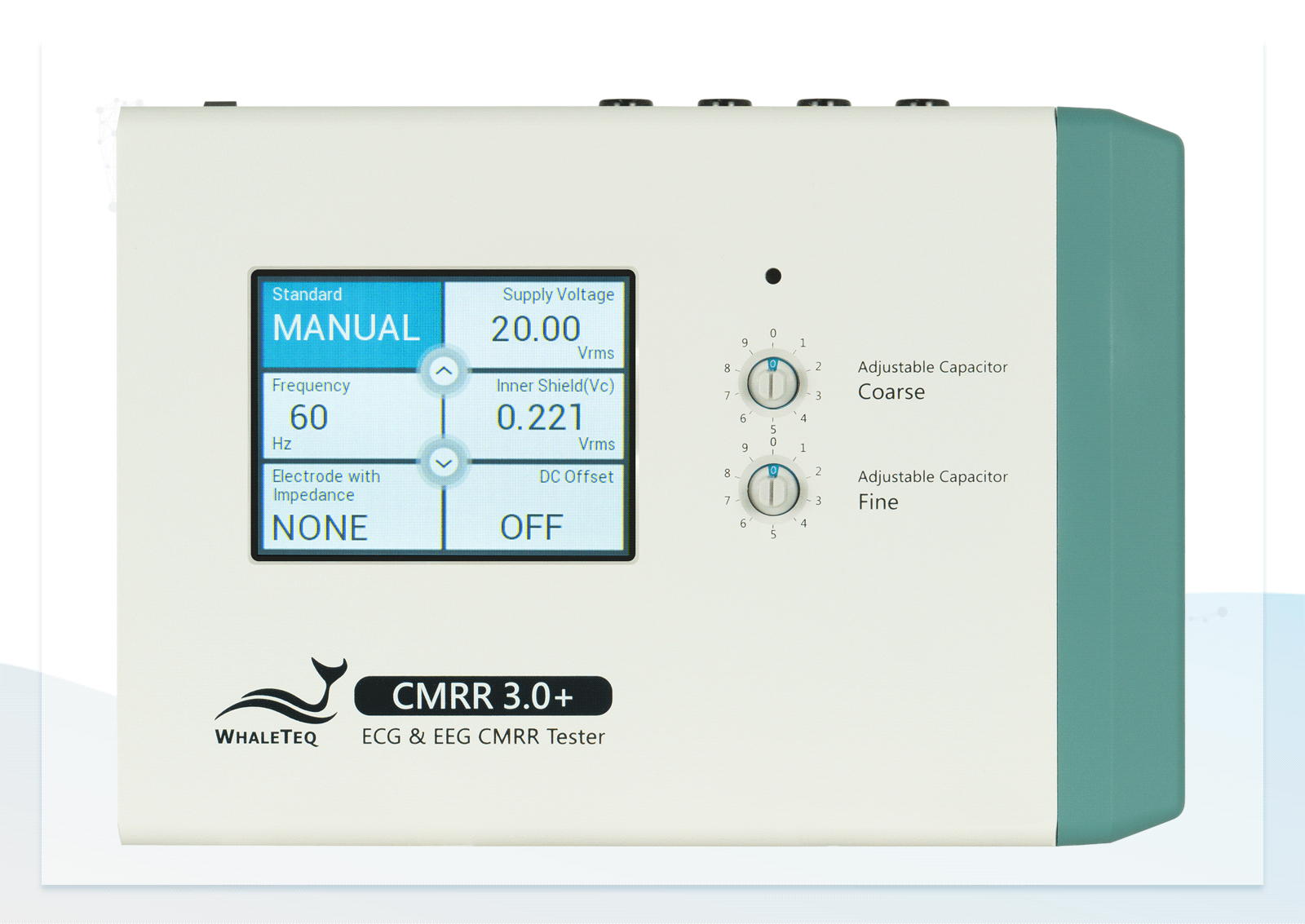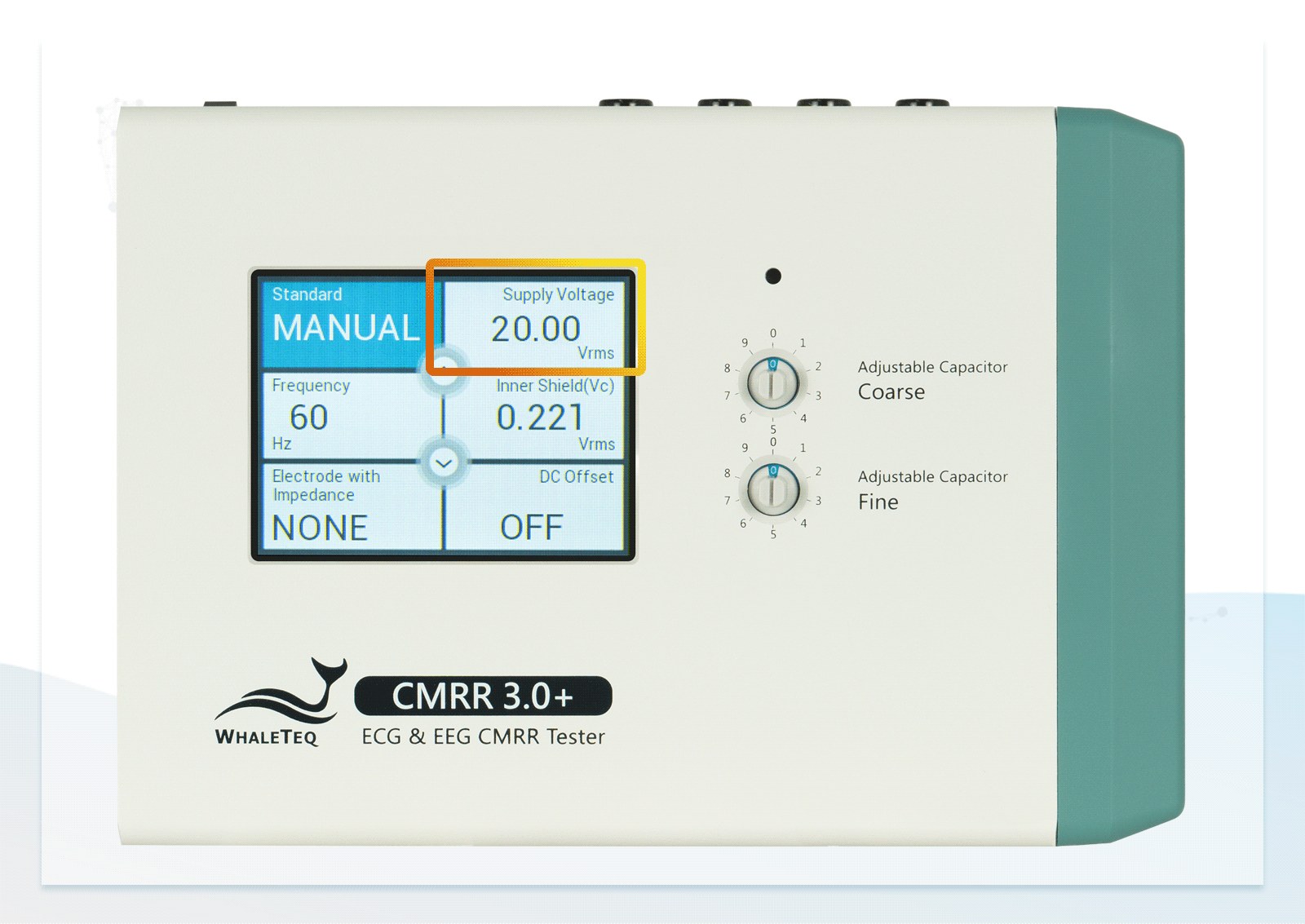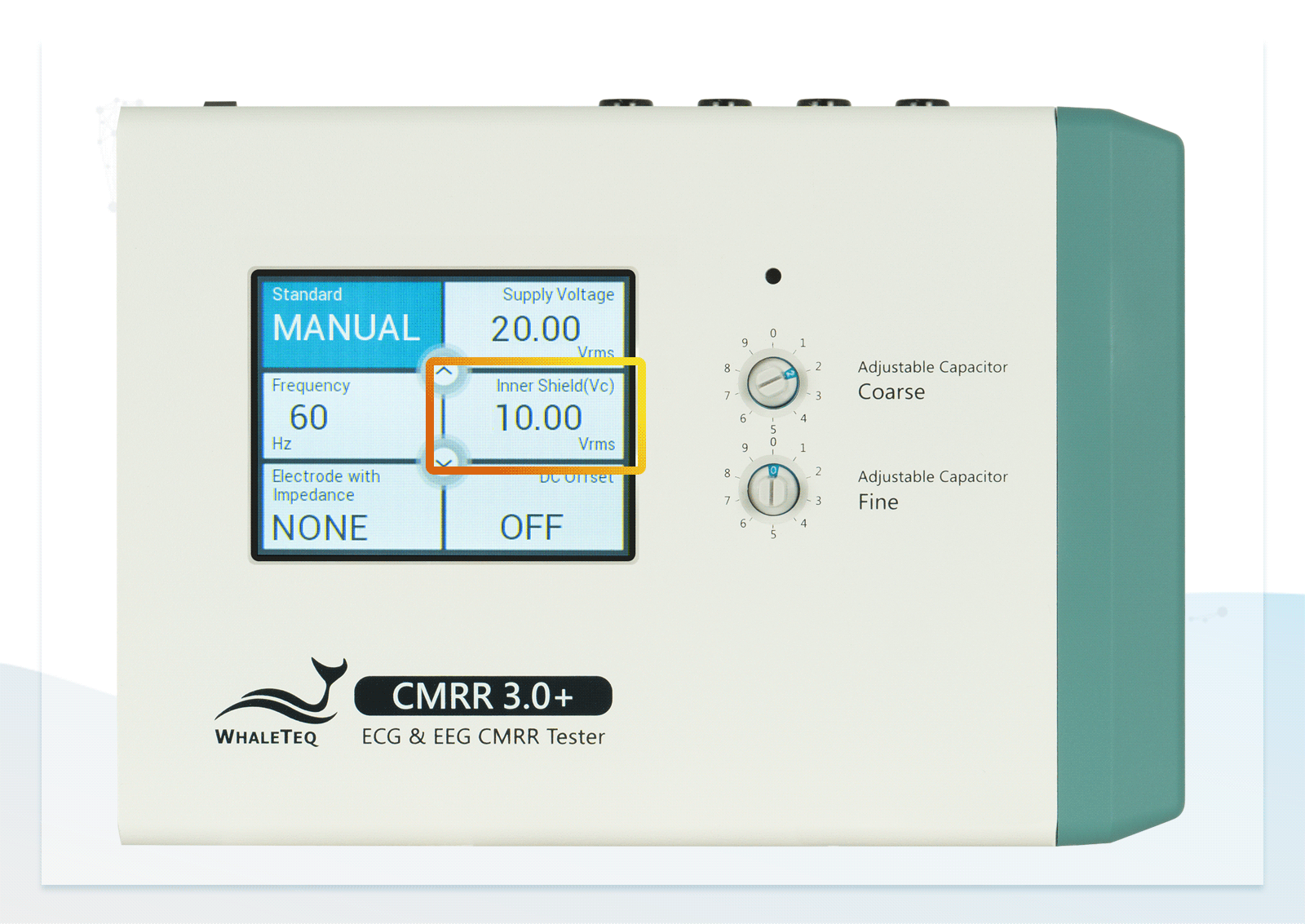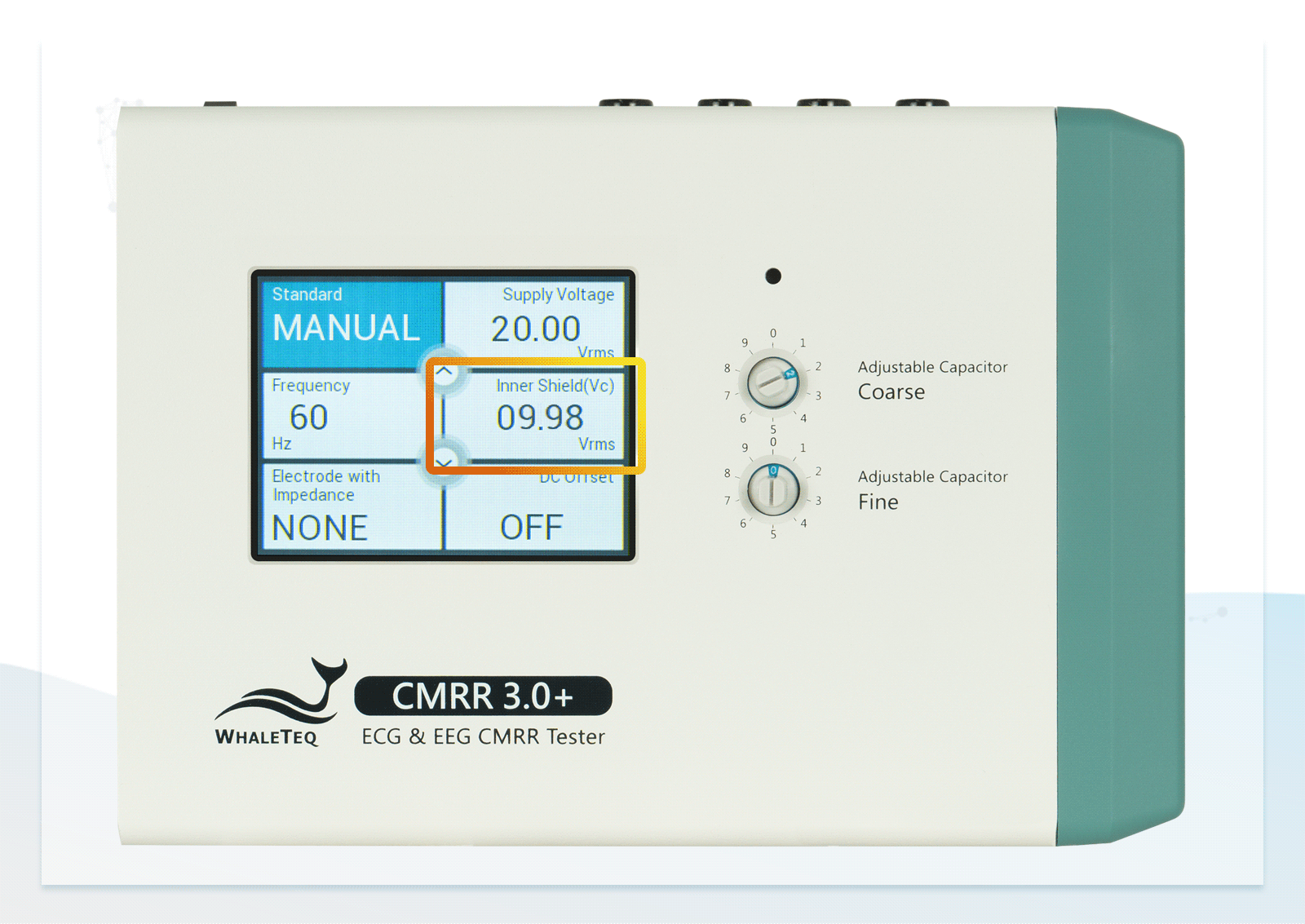


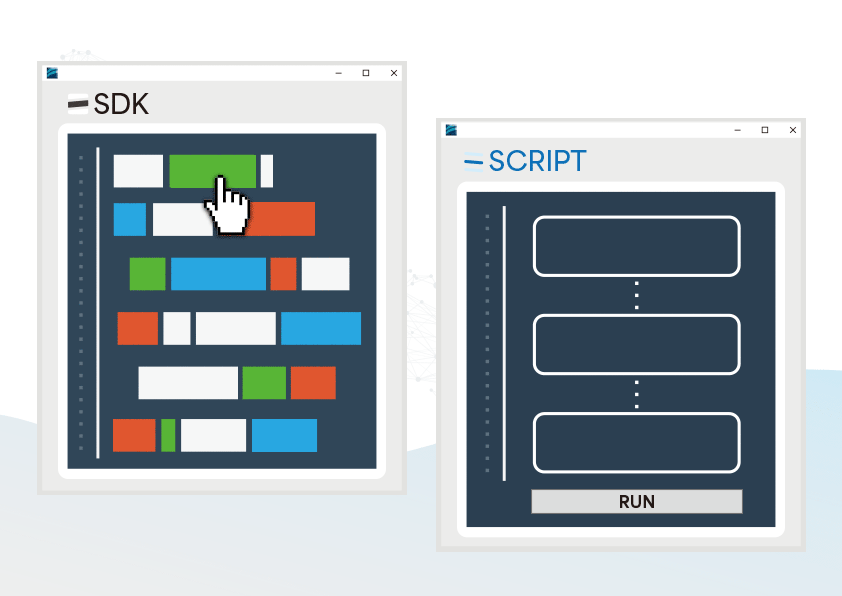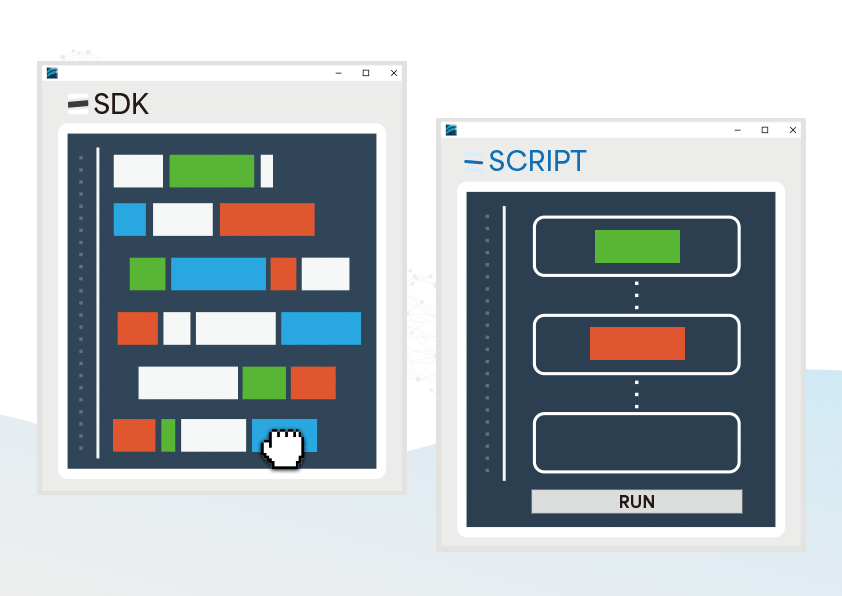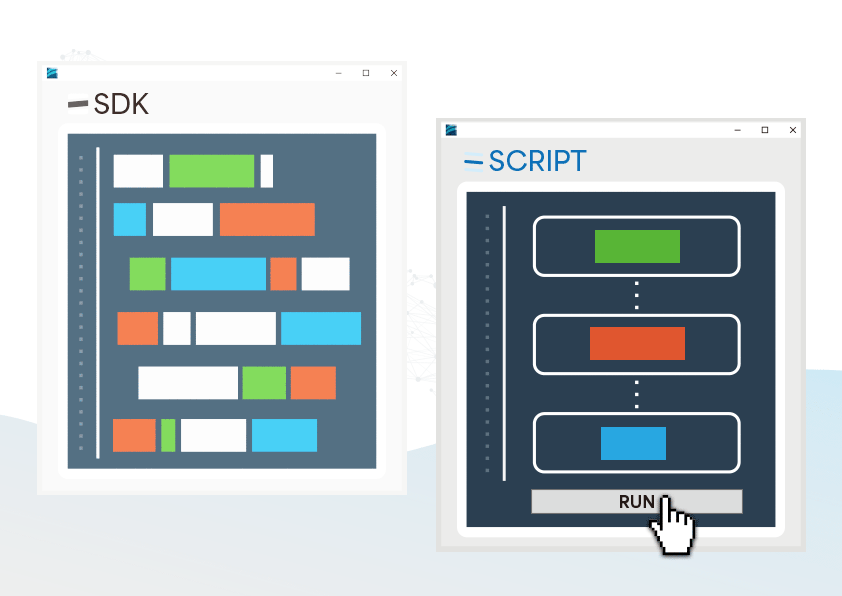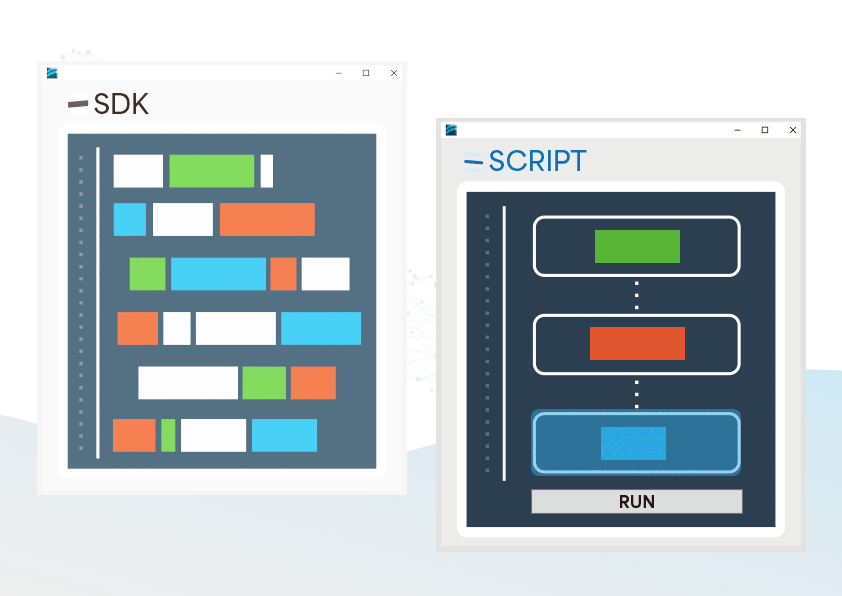





 SDK
SDK

