About WhaleTeq
WhaleTeq designs accurate, easy-to-use, and reliable test solutions for
medical device manufacturers, laboratories, and research institutes.
We focus on vital signs devices, including ECG, health wearables, AED/defibrillators, and UDI (Unique Device Identification System).
4
Industry-first Solutions
Leading Testing Provider of Four Vital Signs
700
Companies
Total Number of Companies Served
36
Countries
Customers’ Countries of Nationality
OUR PRODUCTS
WhaleTeq Co., Ltd. is devoted to developing test solutions and associated services for designated medical standards (IEC 60601-2-X).

Defibrillator / AED Tester
DFS200 | Defibrillator / AED Handheld TesterPerform comprehensive AED testing, capable of checking shock energy waveforms and battery voltage. Paired with a dedicated mobile application connected to the cloud maintenance management platform, it enables real-time tracking and planning of testing routes, facilitating information synchronization and communication between management and inspection personnel.
view more +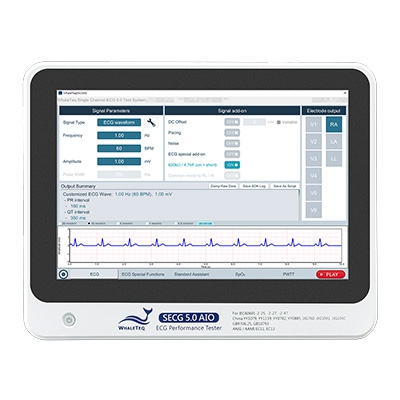
ECG / EKG Testing
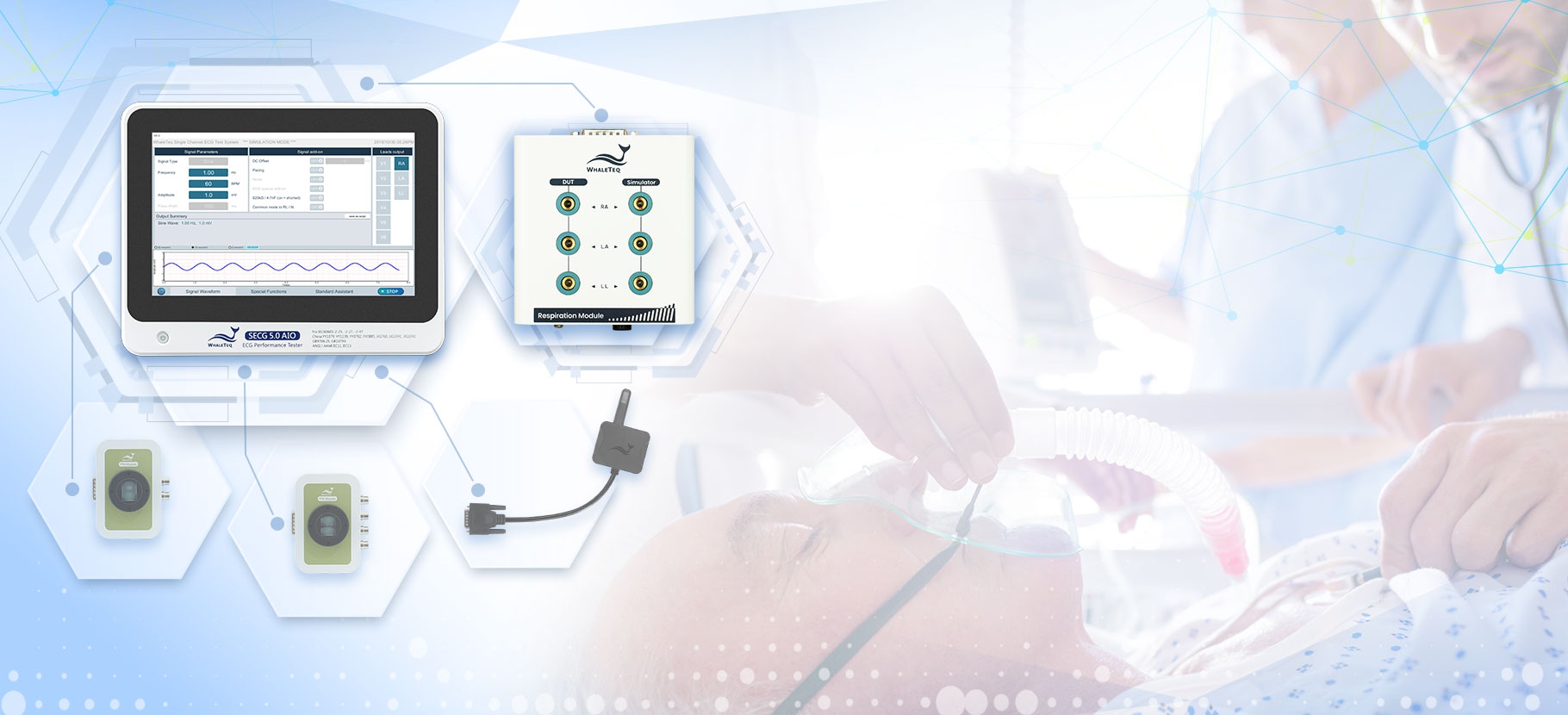
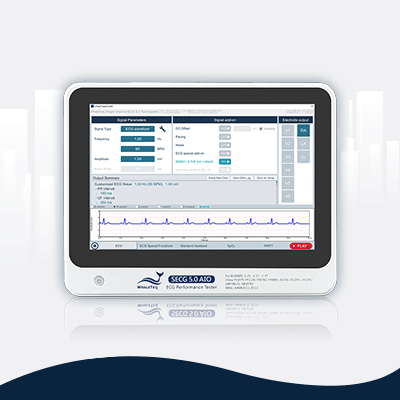
SECG 5.0 AIO | Multi Vital Sign SimulatorDesigned specifically for hardware and system engineers, this standalone multi-vital sign simulator outputs highly accurate and low-noise ECG analog signals. It can superimpose various signals, including respiration, pacing, and noise simulation signals, aiding engineers in performing tests that closely resemble real-world scenarios to effectively verify the performance of the device under test (DUT). When paired with WhaleTeq’s PPG modules, it can simultaneously simulate SpO2 signals for PWTT or PWV testing.
Equipped with the standard assistant software, it enables engineers to conduct compliance tests conforming to the following standards within a few clicks: IEC 60601-2-25, IEC 60601-2-27, IEC 60601-2-47, GB 9706.225, GB 9706.227, YY 9706.247, YY1079, YY1139, YY0782, YY0885, JJG 760, JJG 1042, ANSI/AAMI EC11, and ANSI/AAMI EC13.
view more +Equipped with the standard assistant software, it enables engineers to conduct compliance tests conforming to the following standards within a few clicks: IEC 60601-2-25, IEC 60601-2-27, IEC 60601-2-47, GB 9706.225, GB 9706.227, YY 9706.247, YY1079, YY1139, YY0782, YY0885, JJG 760, JJG 1042, ANSI/AAMI EC11, and ANSI/AAMI EC13.

Health Wearables Testing
AECG100|Wearable ECG & PPG SimulatorThe Innovative simulator to output ECG and PPG analog signals simultaneously. Adjustable time difference parameters to generate various Pulse Wave Transit Time (PWTT) signals. Complies with the latest international medical standards: IEC 60601-2-47, IEC 63203-402-3:2024, YY 0885, YY 9706.247, suitable for R&D, production lines and compliance testing.
view more +
Oximeter Device Testing
PPG-2TF-660 | Transmittance SpO2 ModuleWorks with the AECG100 main console or SECG 5.0 AIO Multi Vital Sign Simulator as the first transmittance SpO2 performance tester designed for R&D engineers in oximeter manufacturers.
This dual-channel optical module outputs R and IR analog signals to test heart rate and blood oxygen saturation (SpO2) functions and is suitable for the development of transmittance PPG products such as finger clip oximeters and patient monitors to test performance.
view more +This dual-channel optical module outputs R and IR analog signals to test heart rate and blood oxygen saturation (SpO2) functions and is suitable for the development of transmittance PPG products such as finger clip oximeters and patient monitors to test performance.

Defibrillator / AED Tester
DFS400 | AED/Defibrillator TesterDesigned for engineers at AED/defibrillator manufacturers, the tester integrates multiple needed testing equipment and functions. With its dedicated software, test data such as the shock waveform, energy (in Joules), parameters, and characteristic points are displayed on one screen and automatically saved to establish a comprehensive test history.
Moreover, it offers switchable loads to evaluate AED shock performance across different scenarios and allows comparison of multiple shock waveforms through the Repeatability and Reproducibility Analysis, ensuring consistent AED/defibrillator quality and reliability.
view more +Moreover, it offers switchable loads to evaluate AED shock performance across different scenarios and allows comparison of multiple shock waveforms through the Repeatability and Reproducibility Analysis, ensuring consistent AED/defibrillator quality and reliability.

Health Wearables Testing
WECG400|Parallel Testing ECG Tester for Wearable Device Production LineThe first parallel testing ECG tester specifically designed for wearable devices can simultaneously test ECG function on up to 4 devices with a single computer to accelerate testing time.
It provides compliance tests conforming with IEC 60601-2-47, YY0885, and YY 9706.247-2021, and can output 7 types of standard ECG waveforms to assist wearable devices in meeting regulatory requirements of accuracy.
view more +It provides compliance tests conforming with IEC 60601-2-47, YY0885, and YY 9706.247-2021, and can output 7 types of standard ECG waveforms to assist wearable devices in meeting regulatory requirements of accuracy.

Oximeter Device Testing
PPG-2R-880 / PPG-2R-940 | Reflectance SpO2 ModuleThe PPG-2R-880/PPG-2R-940 works with the AECG100 main console or SECG 5.0 AIO Multi Vital Sign Simulator, as the first reflectance SpO2 performance tester designed for R&D in manufacturers.
This dual-channel optical module outputs R and IR analog signals to test blood oxygen saturation (SpO2) and heart rate functions. Besides, it also allows users to adjust the R/IR’s rate of change of respiratory signals in PPG and set up add-on noise. It’s suitable for the development and verification of reflectance PPG products like wearable devices.
view more +This dual-channel optical module outputs R and IR analog signals to test blood oxygen saturation (SpO2) and heart rate functions. Besides, it also allows users to adjust the R/IR’s rate of change of respiratory signals in PPG and set up add-on noise. It’s suitable for the development and verification of reflectance PPG products like wearable devices.

Defibrillator / AED Tester
AED CMMS PLATFORMSimplify your AED Inspection and Complete Testing
A unified and cost-effective AED Management System with up-to-date reminder, test report, status tracking, and device management. Also, it provides complete testing solution includes on-site inspection, shock energy test, and battery test, which helps you lower the legal risk of AED ownership.
view more +A unified and cost-effective AED Management System with up-to-date reminder, test report, status tracking, and device management. Also, it provides complete testing solution includes on-site inspection, shock energy test, and battery test, which helps you lower the legal risk of AED ownership.
Find A Solution
END
NO DATA
NEWS & EVENT

Company News
2024 / 10 / 30
Join WhaleTeq at the 2024 Global Conference on Biomedical Engineering in Tainan!

Company News
2024 / 09 / 27
WhaleTeq Has Been Authorized to Market the CTS Database in Cooperation with Corscience GmbH & Co. KG for Enhancing ECG Compliance Testing Solutions

Product News
2024 / 06 / 28
WhaleTeq SECG 5.0 AIO Functionality Enhanced! Expanded Respiratory Signal Simulation Range and Optimized Resolution with the New Respiration Module

Company News
2024 / 04 / 30
WhaleTeq AECG100, HRS200, and HRS100+ test equipment support the newly released international standard for wearable devices, IEC 63203-402-3:2024

Company News
2024 / 04 / 12
WhaleTeq's First-Time Presence at Medtec Japan, Asia's Largest Medical Device Exhibition

Company News
2024 / 04 / 03
Join WhaleTeq at CMEF China Medical Equipment Fair!
Company News
2024 / 02 / 22
WhaleTeq Leads the Innovation in Blood Pressure Monitors by Improving the Accuracy of Medical Devices

Company News
2024 / 01 / 19
WhaleTeq cordially invites you to visit our booth at 10th WEARABLE EXPO

Company News
2023 / 10 / 25
Explore Vital Sign Simulation with WhaleTeq!

Product News
2023 / 09 / 25
WhaleTeq enhances the SECG 5.0 AIO with reflectance PPG modules, offering PWTT testing for wearable devices’ blood pressure measurement algorithms

Company News
2023 / 09 / 14
WhaleTeq's Debut at EMS World EXPO, Join Us in Caring for Emergency Medical Services

Product News
2023 / 08 / 11
WhaleTeq introduces the DFS400, a dedicated tester for AEDs, ensuring quality and reliability for manufacturers
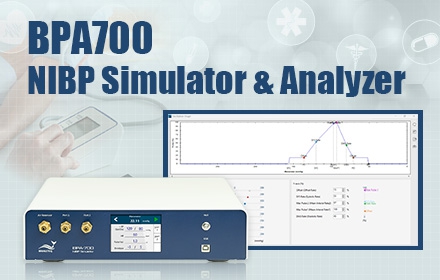
Product News
2023 / 07 / 28
WhaleTeq release the revolutionary new product BPA700, altering the current blood pressure monitor testing

Company News
2023 / 07 / 20
Achieve Mutual Success with WhaleTeq!

Company News
2023 / 06 / 01
【WhaleTeq 10th Anniversary】Exclusive Rewards for Calibration Services

Product News
2023 / 05 / 15
WhaleTeq unveils the upgraded SECG 5.0 AIO, collaborating with PPG-2TF-660 to effectively validate ECG, SpO2, and PWTT performance of patient monitors

Company News
2023 / 04 / 20
WhaleTeq Takes Part in Secutech 2023 for the First Time!

Company News
2023 / 04 / 12
WhaleTeq Invites You to Visit Us on Chongqing International Medical Device Exhibition

Company News
2023 / 03 / 22
WhaleTeq will attend KiMES 2023 from 23rd~ 26th, Korea's Largest Medical and Hospital Equipment Show

Company News
2023 / 02 / 17
Reminder Regarding Information Security

Product News
2022 / 11 / 28
WhaleTeq launches the WECG400, a first-ever parallel testing ECG tester designed for the wearable device production line to improve efficiency

Company News
2022 / 09 / 30
WhaleTeq will attend MEDICA 2022 in Düsseldorf, Germany

Product News
2022 / 07 / 15
WhaleTeq launches the AED NB-IoT Module WAIOT-N for remotely monitoring the status of AEDs

Company News
2021 / 11 / 22
WhaleTeq cordially invites you to visit our booth (N427a) at Healthcare+ Expo Taiwan 2021!

Product News
2021 / 11 / 19
WhaleTeq Launches DFS360 Defibrillator/AED Production Line Tester

Company News
2021 / 10 / 07
WhaleTeq will be joining 85th China International Medical Equipment Fair

Company News
2021 / 10 / 04
Let's meet at MEDICARE TAIWAN 2021
USER TESTIMONIALS
![[Schiller] Jean, Philippe DIDON, PhD, Schiller Medical](data/witness/cover/1676605782973958692.png)
Using WhaleTeq's tools allowed us to be more efficient during development and evaluation phase of our ECG related modules.
[Schiller] Jean, Philippe DIDON, PhD, Schiller Medical
Read the full testimonial
![[CSEM] Patrick Theurillat - Patrick Theurillat, PPG Technology expert and Richard Delgado Gonzalo, Head of Embedded Software](data/witness/cover/1676605724709962477.png)
We are looking forward to see the next generation Whaleteq test devices and to continue using AECG100 to successfully get new products to the market.
[CSEM] Patrick Theurillat - Patrick Theurillat, PPG Technology expert and Richard Delgado Gonzalo, Head of Embedded Software
Read the full testimonial
![[AliveCor] Lauren Meleney, Engineering Program Manager, AliveCor, Inc.](data/witness/cover/1676605466752471894.png)
I am writing this letter to recommend the WhaleTeq AECG100 Test System. Using WhaleTeq tools allows us to test efficiently and confidently.
[AliveCor] Lauren Meleney, Engineering Program Manager, AliveCor, Inc.
Read the full testimonial
![[Corscience] Dr. Tobias Tröger, Head of Research and Development, Corscience GmbH & Co. KG](data/witness/cover/1676605648540309631.png)
With the WhaleTeq solutions our team is able to validate our products in a very efficient way, because using the automated test sequences saves a lot of time. Thanks very much for these great products!
[Corscience] Dr. Tobias Tröger, Head of Research and Development, Corscience GmbH & Co. KG
Read the full testimonial
![[Corpuls] Dr. Antoun Khawaja, Director Signal Processing, GS Elektromedizinische Geräte G. Stemple GmbH](data/witness/cover/1676605567790736711.png)
I can recommend the WhaleTeq SDK as a helpful tool for the validation of ECG products.
[Corpuls] Dr. Antoun Khawaja, Director Signal Processing, GS Elektromedizinische Geräte G. Stemple GmbH
Read the full testimonial
![[NeuroSky] Jerry Kuo, Analog IC Design Manager, NeuroSky Inc.](data/witness/cover/1676605368692830934.png)
WhaleTeq provides very useful products for us on ECG verification, which can accelerate our chip development.
[NeuroSky] Jerry Kuo, Analog IC Design Manager, NeuroSky Inc.
Read the full testimonial